Team:DTU-Denmark/Experiment4
From 2013.igem.org
(Created page with "{{:Team:DTU-Denmark/Templates/StartPage|Aerobic consumption of ammonium and hydroxylamine}} == Introduction == == Methods == [[Team:DTU-Denmark/Methods/Determining_concentrati...") |
|||
| Line 2: | Line 2: | ||
== Introduction == | == Introduction == | ||
| + | |||
| + | In this aerobic experiment, we add ammonium NH<sub>4</sub><sup>+</sup> to transformed ''E. coli'' cells and measure the consumption of ammonium to determine whether our AMO transformant is converting ammonium to hydroxylamine (NH<sub>2</sub>OH) faster than an untransformed control. A similar protocol is used to determine whether our HAO transformant is converting hydroxylamine to nitrite NO<sub>2</sub><sup>-</sup>, by measuring production of nitrite. | ||
== Methods == | == Methods == | ||
| Line 7: | Line 9: | ||
[[Team:DTU-Denmark/Methods/Determining_concentration_of_nitrogen_compounds/Experiment_4|Experimental methods]] | [[Team:DTU-Denmark/Methods/Determining_concentration_of_nitrogen_compounds/Experiment_4|Experimental methods]] | ||
| - | == Conclusion == | + | == Results == |
| + | |||
| + | [[File:Dtu-rate-amo.png|600px]] | ||
| + | |||
| + | == Conclusion and Discussion == | ||
| + | |||
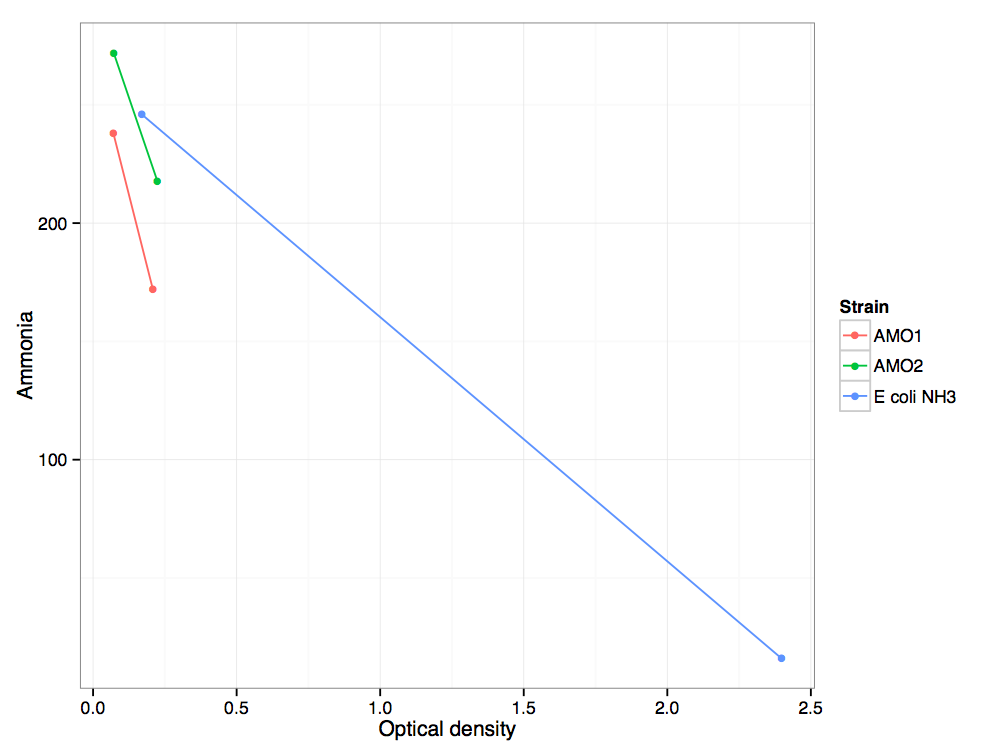
| + | The AMO transformant uses ammonia at a rate greater than the untransformed ''E. coli'' control. This is due to the AMO consuming ammonia and converting it to hydroxylamine. Additionally, however, the AMO transformant grows significantly more slowly than the control. | ||
| - | |||
| + | It is clear that hydroxylamine is toxic to the cells at this concentration. With more time, it would be interesting to repeat the [[Team:DTU-Denmark/ToxicityExperiment|toxicity experiment]] using hydroxylamine to determine the threshold concentration that causes cell death. We expect that ''in vivo'', the hydroxylamine to nitrite reaction catalyzed by HAO | ||
| + | happens very quickly (TODO add ref) and that even at high throughput, hydroxylamine should not accumulate in the cell. | ||
<div style="clear: both;"></div> | <div style="clear: both;"></div> | ||
{{:Team:DTU-Denmark/Templates/EndPage}} | {{:Team:DTU-Denmark/Templates/EndPage}} | ||
Revision as of 20:56, 29 September 2013
Aerobic consumption of ammonium and hydroxylamine
Contents |
Introduction
In this aerobic experiment, we add ammonium NH4+ to transformed E. coli cells and measure the consumption of ammonium to determine whether our AMO transformant is converting ammonium to hydroxylamine (NH2OH) faster than an untransformed control. A similar protocol is used to determine whether our HAO transformant is converting hydroxylamine to nitrite NO2-, by measuring production of nitrite.
Methods
Results
Conclusion and Discussion
The AMO transformant uses ammonia at a rate greater than the untransformed E. coli control. This is due to the AMO consuming ammonia and converting it to hydroxylamine. Additionally, however, the AMO transformant grows significantly more slowly than the control.
It is clear that hydroxylamine is toxic to the cells at this concentration. With more time, it would be interesting to repeat the toxicity experiment using hydroxylamine to determine the threshold concentration that causes cell death. We expect that in vivo, the hydroxylamine to nitrite reaction catalyzed by HAO
happens very quickly (TODO add ref) and that even at high throughput, hydroxylamine should not accumulate in the cell.
 "
"