Team:DTU-Denmark/Experiment4
From 2013.igem.org
(→Results) |
(→Results) |
||
| Line 12: | Line 12: | ||
[[File:Dtu-rate-amo.png|600px]] | [[File:Dtu-rate-amo.png|600px]] | ||
| + | |||
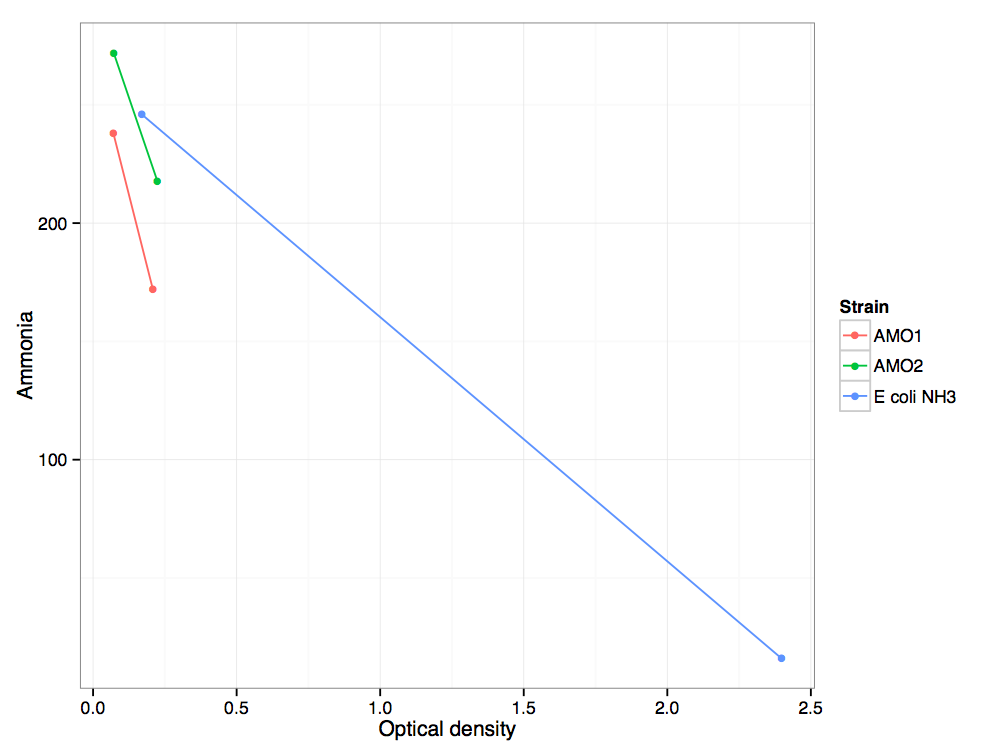
Ammonia measured plotted vs optical density from minutes 129 to 885 for two replicates of the AMO transformant, and an untransformed ''E. coli'' control. | Ammonia measured plotted vs optical density from minutes 129 to 885 for two replicates of the AMO transformant, and an untransformed ''E. coli'' control. | ||
Revision as of 21:00, 29 September 2013
Aerobic consumption of ammonium and hydroxylamine
Contents |
Introduction
In this aerobic experiment, we add ammonium NH4+ to transformed E. coli cells and measure the consumption of ammonium to determine whether our AMO transformant is converting ammonium to hydroxylamine (NH2OH) faster than an untransformed control. A similar protocol is used to determine whether our HAO transformant is converting hydroxylamine to nitrite NO2-, by measuring production of nitrite.
Methods
Results
Ammonia measured plotted vs optical density from minutes 129 to 885 for two replicates of the AMO transformant, and an untransformed E. coli control.
Conclusion and Discussion
The AMO transformant uses ammonia at a rate greater than the untransformed E. coli control. This is due to the AMO consuming ammonia and converting it to hydroxylamine. Additionally, however, the AMO transformant grows significantly more slowly than the control.
It is clear that hydroxylamine is toxic to the cells at this concentration. With more time, it would be interesting to repeat the toxicity experiment using hydroxylamine to determine the threshold concentration that causes cell death. We expect that in vivo, the hydroxylamine to nitrite reaction catalyzed by HAO
happens very quickly (TODO add ref) and that even at high throughput, hydroxylamine should not accumulate in the cell.
 "
"