Team:DTU-Denmark/Notebook/1 August 2013
From 2013.igem.org
1 August 2013
Contents |
lab 208
Main purpose
- Gel purification of HAO
- PCR on single Nir fragments also with PAO1 sample from environmental lab
- USER reaction
Who was in the lab
Kristian, Natalia, Julia, Henrike
Procedure
gel purification
Gel purified HAO fragment for USER cloning and pZA21 with endings for cloning with the 2-part Nir. Used QIAEX kit (without columns).
PCR
Set up PCR to amplify AMO as fragment for USER cloning. Run triplicates on 50C and 3:00 and dublicates on a ramp program 56C -> 50C with 0.1C/s and 3:00
USER reaction with HAO and pZA21 (native)
Per reaction:
- USER enzyme - 1 uL
- NEB buffer 4 - 0.5 uL
- 10x BSA - 0.5 uL
- backbone - 1 uL
- fragment - 7 uL
Run reaction for 40 min on 37C and 30 mins on 25C. Made doubles and negative. One reaction is incubated with 50 uL of Top10 competent cells, the other with 100 uL of our competent cells. Only heat shock Top10 for 30 seconds, the other for 90 sec. Incubation for 2 h with 400 uL of SOC, afterwards plating on 30ug/mL kanamycin plates. Cells will be grown on the bench instead of in the incubator.
LB+Kan plates
Made agar plates from LB medium and added Kanamycin to final concentration of 30 ug/ml.
Rehydration of primers
Received new primers. Rehydration with 150uL MilliQ to obtain stock solution and store in -80. 10uL of stock solution mixed with 90 uL of MilliQ to obtain -20 working solution.
PCR on Nir and single fragment Nir
All Nir PCR's where done with Touch down PCR going from 71C → 64C increment of -1C each cycle in 14 rounds and afterwards 25 cycles at 64C. The extension time was calculated as 20 to 30 sec per 1kb. Nir E is with environmental lab sample PAO1. Nir G is with genomic dna isolated from PAO1 from 301. Nir H is with genomic dna isolated from PAO1 and treated with HindIII from 301. If not otherwise stated the template is environmental lab sample PAO1.
The number tells which primer pair we used. The following are the reactions
- Nir E with 11, 41,42, 43, 44 and 48 respectively.
- Nir G with 43, 44, 48 respectively.
- Nir H with 43, 44, 48 respectively.
- Neg. control
PCR on cycAX with his-tag primers
2x samples of cycAX with primer pair 33 on PCR with 10 cycles of 62C and afterwards 25 cycles of 70C. Extension time was 3:30 min.
Results
Gels
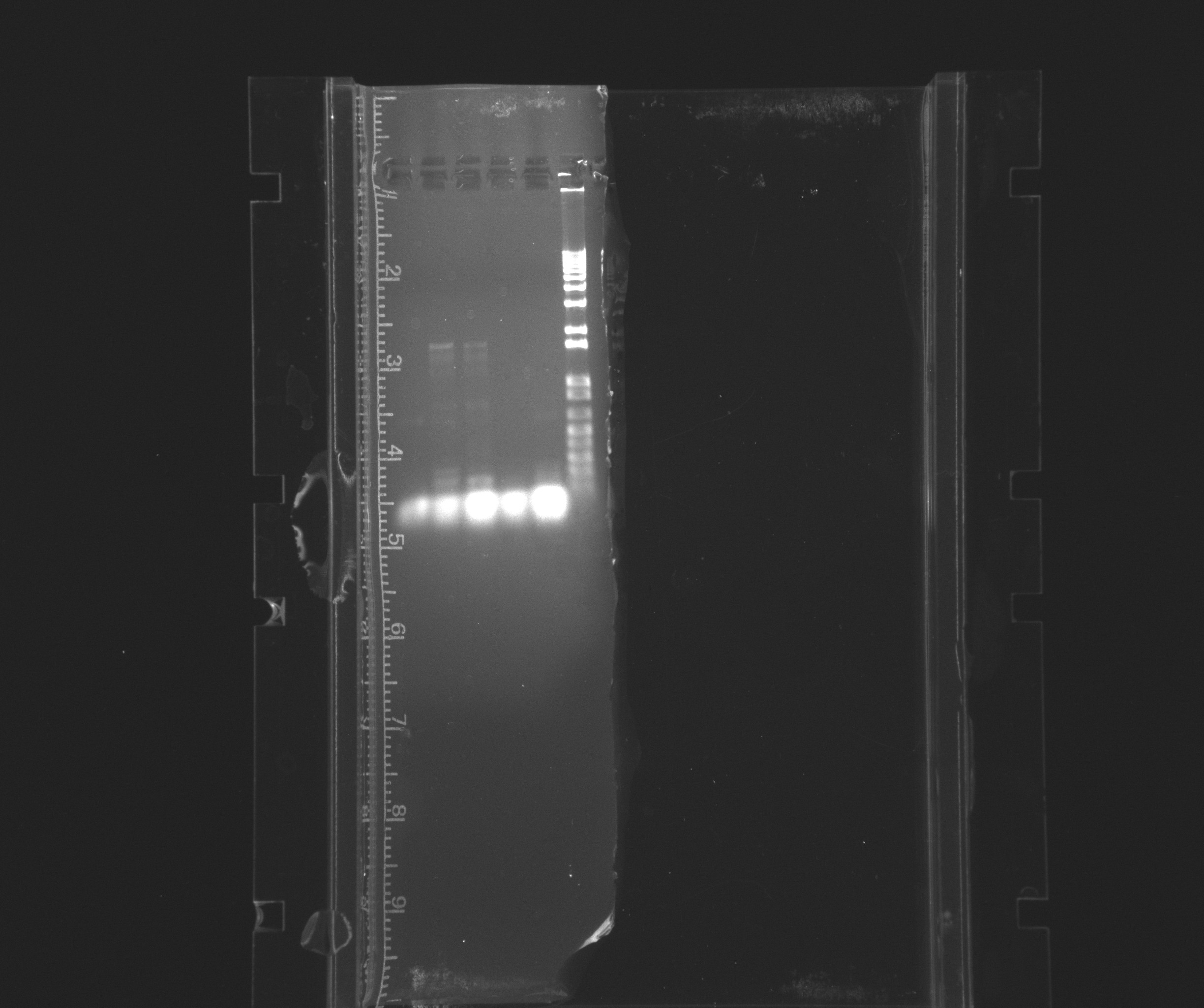
1% gel for PCR of cytochromes with His-tag
- neg
- cycAX with His-tag at 45C
- cycAX with His-tag at 45C
- cycAX with His-tag at 50C
- cycAX with His-tag at 50C
- 1 kb ladder
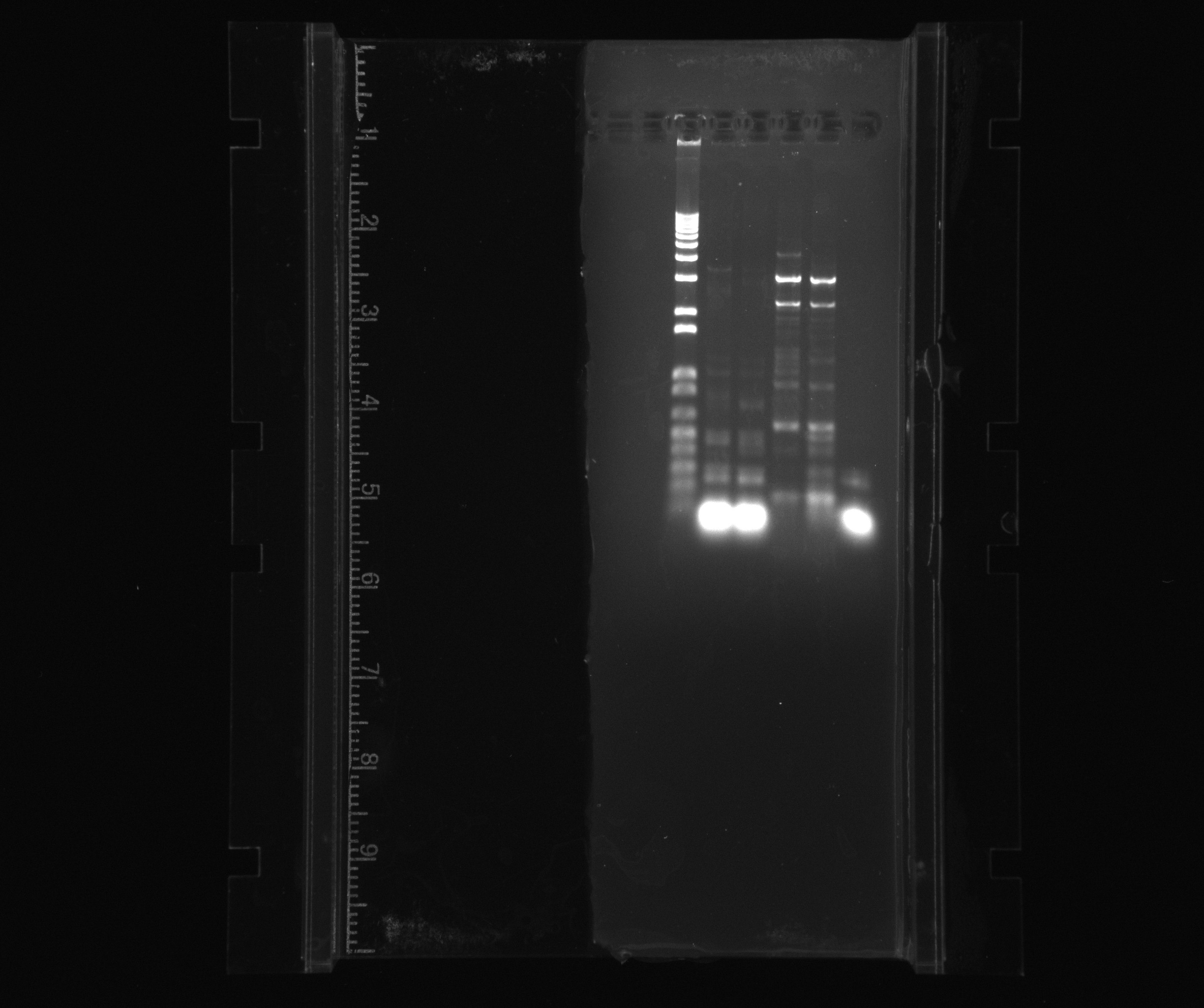
1% gel for PCR products of Nir
- 1 kb ladder
- Nir part 2
- Nir part 2
- Nir part 1
- Nir part 1
- neg
Conclusion
Navigate to the Previous or the Next Entry
 "
"