Team:DTU-Denmark/Notebook/22 August 2013
From 2013.igem.org
(→Main purpose) |
(→Procedure) |
||
| Line 104: | Line 104: | ||
<hr/> | <hr/> | ||
| - | Adjusting the temperature at 37 degrees and calibrating the probes as described in | + | Adjusting the temperature at 37 degrees and calibrating the probes as described in [[Team:DTU-Denmark/Methods/Calibrating_Electrodes|Calibration]]. |
Revision as of 17:45, 25 August 2013
22 August 2013
Contents |
lab 208
Main purpose
Who was in the lab
Kristian, Henrike
Procedure
USER ligation and transformation
Redid for HAO and cyc in arabinose inducible pZA21::araBAD and for Nir fragments
PCR for Biobrick parts
Set up a new PCR reaction for Biobrick parts using HF buffer and 5% DMSO but no MgCl2. PCR was run on a touchdown program
primers: 53a, 53b
template: Sec2 miniprep
program:
| temperature | time | cycles |
|---|---|---|
| 98C | 2:00 | - |
| 98C | 0:10 | 10 |
| 63C | 1:00 | 10 |
| -0.5C per cycle | ||
| 72C | 1:00 | 10 |
| 98C | 0:10 | 25 |
| 53C | 1:00 | 25 |
| 72C | 1:00 | 25 |
| 72C | 5:00 | - |
| 10C | hold | - |
Gel purification
Gel purified the linearized plasmid pZA21::ara with USER endings
colony pPCR to verify AMO insert
Picked 10 colonies from yesterday's cloning for colony PCR. Diluted cells in 50 uL of MilliQ and took 1 uL as template
primers: 53a, 53b
program:
| temperature | time | cycles |
|---|---|---|
| 98C | 10:00 | - |
| 98C | 0:10 | 36 |
| 56C | 0:30 | 36 |
| 72C | 2:00 | 36 |
| 72C | 5:00 | - |
| 10C | hold | - |
Results
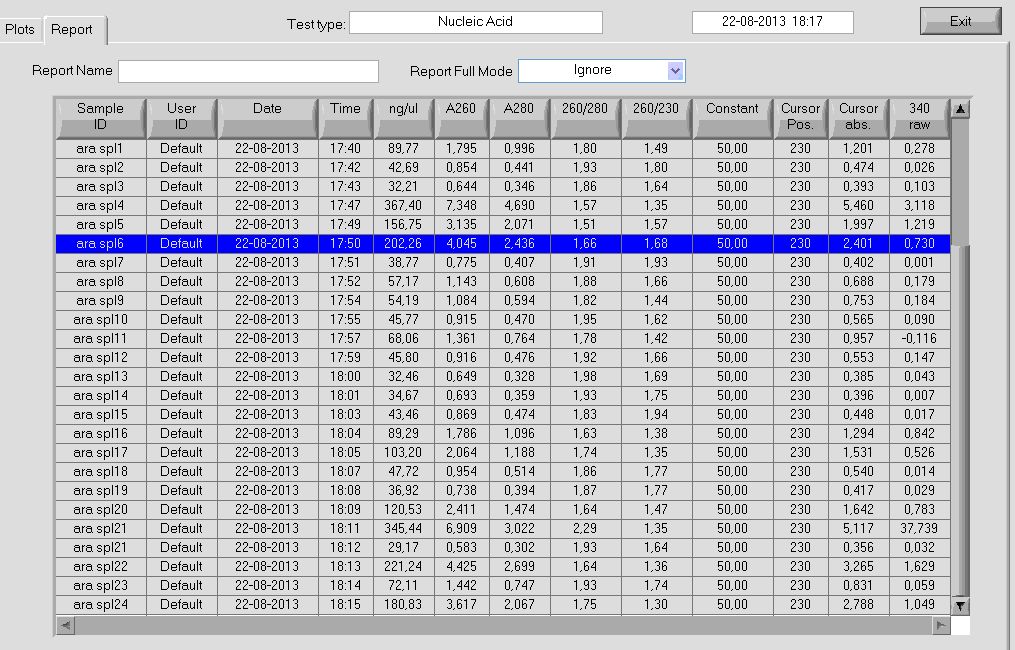
nanodrop measurement of ara spl plasmids
Conclusion
lab 115
Main purpose
Run Experiment 2 in two different samples anaerobically in order to characterize the behavior of E.coli.
Who was in the lab
Ariadni, Helen
Procedure
Adjusting the temperature at 37 degrees and calibrating the probes as described in Calibration.
Following the protocol Experiment 2
Changing the steps :
6. 3 ml of the overnight culture (from glycerol stock) in 200 ml of medium with OD=0.0317.
7. Then added 3 ml more after 2 hours and the OD was 0.1046. The OD after 1 hour was 0.1340. Afterwards we added glycerol 1g in 10 ml of water and then added in our growing culture. Then after 40 min the OD was 0.2737. (Setup wavelength 600 nm).
12. The OD was measured 0.1324 instead of 0.3.
19. Add 0.5 ml of nitrite solution and continue by adding 0.5 ml after 10 minutes then we took 2.2 ml of sample, we added then 1 ml and we saw a slow response after 6 minutes. Afterwards when there was any change in the curve we spiked with 1 ml of nitrite. We continued as there was no response by adding 1 ml more and then by adding 2 ml more. We took 2 ml for sample in the end and another 2 ml for OD measurement where OD=0.1350. One hour after we took 2 ml of sample and then we spiked with 1 ml of NO2. There was no response after 12 minutes. Then we spiked with 0.5 ml of N2O and we show a big response.
Results
Colorimetric results
Ammonium
Measuring range 2-75 mg/L NH4-N
start point- signal <2 mg/L
middle point- signal 2 mg/L
end point- signal <2 mg/L
Nitrate
Measuring range 1-25 mg/L NO3-N
start point- signal <1 mg/L
middle point- signal <1 mg/L but visible pinker
end point- signal <1 mg/L but much visible pinker
Nitrite
Measuring range 0.02-1 mg/L NO2-N
start point- signal <0.02 mg/L
middle point- signal 0.29 mg/L
end point- signal 0.70 mg/L after X2 dilution
Point before spiking with NO2 at the end
measurement 1.03 mg/L (x2 dilution)- 0.5 mg/L (x4 dilution)
Conclusion
The E.coli didn't grow as usual and the problem might be the minimal medium.
Navigate to the Previous or the Next Entry
 "
"