Team:DTU-Denmark/Notebook/6 August 2013
From 2013.igem.org
(→Main purpose) |
|||
| Line 6: | Line 6: | ||
==Main purpose== | ==Main purpose== | ||
<hr/> | <hr/> | ||
| + | 1.3.1 PCR for AMO | ||
| + | 1.3.2 PCR for SPL (synthetic promoter library) | ||
| + | 1.3.3 PCR for reference promoter | ||
| + | 1.3.4 PCR for araBAD K808000 | ||
| + | 1.3.5 PCR to extract Nir2 from P. aeruginosa | ||
| + | 1.3.6 PCR for araBAD and SPL with DMSO | ||
| + | 1.3.7 PCR on Nir1 with MgCl2 gradient | ||
| + | 1.3.8 Gel purification | ||
| + | 1.3.9 Plasmid miniprep | ||
==Who was in the lab== | ==Who was in the lab== | ||
Revision as of 09:45, 26 August 2013
6 August 2013
Navigate to the Previous or the Next Entry
Contents |
Lab 208
Main purpose
1.3.1 PCR for AMO 1.3.2 PCR for SPL (synthetic promoter library) 1.3.3 PCR for reference promoter 1.3.4 PCR for araBAD K808000 1.3.5 PCR to extract Nir2 from P. aeruginosa 1.3.6 PCR for araBAD and SPL with DMSO 1.3.7 PCR on Nir1 with MgCl2 gradient 1.3.8 Gel purification 1.3.9 Plasmid miniprep
Who was in the lab
Kristian, Gosia, Natalia, Henrike
Procedure
PCR for AMO
Made four reactions using 1 uL respectively 10 uL of culture from the -20 or from the glycerol stock (-80) as template and adjusted volume of water accordingly.
Primers 10a, 10b.
PCR for SPL (synthetic promoter library)
template: pZa21 with RFP, primers: 52a, 52b1, temperature: 50C, time: 2:30
PCR for reference promoter
template: pZa21 with RFP, primers: 52a, 52b2, temperature: 50C, time: 2:30
PCR for araBAD K808000
template: K808000, primers: 12a, 12bn, temperature: 50C, time: 2:30
PCR to extract Nir2 from P. aeruginosa
PCR for araBAD and SPL with DMSO
Template and primers as previous.
PCR on Nir1 with MgCl2 gradient
Used previous isolation from a gel purification to make PCR for amplification of the strand. Used 5% DMSO as additive. The PCR was done with primer pair 41 and with x7 polymerase at 55C annealing and 5:00 min extension. Also we used 20 sec as denaturing time instead of the normal 10. The magnesium gradient was as follow:
- 0
- 1uL MgCl2 2mM per 50uL reaction
- 5uL MgCl2 2mM per 50uL reaction
- 20uL MgCl2 2mM per 50uL reaction
Gel purification
of the Nir1 fragment extraced from P. aeruginosa. Nanodrop: 8ng/uL
Plasmid miniprep
HAO in pZA21 from USER cloning was purified for restriction analysis. Nanodrop measurement: 180ng/uL
Results
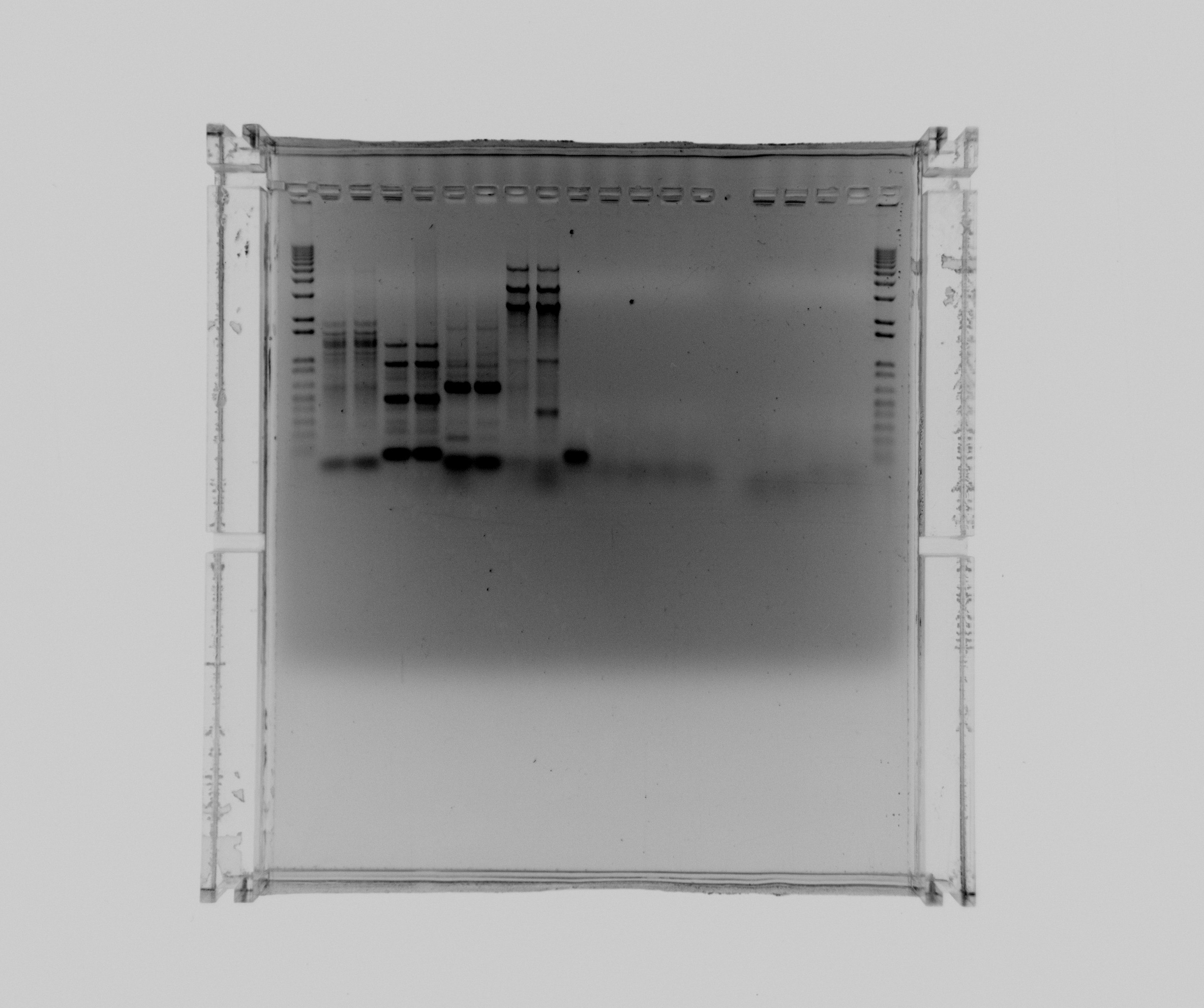
Gel 1
1% agarose gel
- 1 kb ladder
- Nir1 col. Taq
- Nir1 col. Taq
- Nir2 col. Taq
- Nir2 col. Taq
- Nir1 frag. Taq
- Nir1 frag. Taq
- Nir1 col. x7
- Nir1 col. x7
- neg
- Hi spec. buffer
- Hi spec. buffer
- DMSO
- DMSO
- Nir1
- Nir1
- Nir2
- Nir2
- 1 kb ladder
Nir1 was purified.
Gel 2
1% agarose gel
- 1 kb ladder
- NirW
- NirW
- araBAD biobrick K808000
- araBAD biobrick K808000
- SPL in pZA21 containing RFP
- SPL in pZA21 containing RFP
- reference promoter in pZA21 containing RFP
- reference promoter in pZA21 containing RFP
- neg
- neg
- 1 kb ladder
Gel 3
1% agarose gel
- 1 kb ladder
- AMO, 1uL of -20 culture as template
- AMO, 10uL of -20 culture as template
- AMO, 1uL of glycerol stock as template
- AMO, 10uL of glycerol stock as template
- neg from AMO PCR
- 1 - SPL, primers 52a, 52b1, template pZA21 with RFP, 50C, 2:30 min extension
- 2 - SPL, primers 52a, 52b1, template pZA21 with RFP, 50C, 2:30 min extension
- 3 - ref, primers 52a, 52b2, template pZA21 with RFP, 50C, 2:30 min extension
- 4 - ref, primers 52a, 52b2, template pZA21 with RFP, 50C, 2:30 min extension
- 5 - araBAD, primers 12a, 12bn, template K80800, 50C, 2:30 min extension
- 6 - araBAD, primers 12a, 12bn, template K80800, 50C, 2:30 min extension
- 1 kb ladder
AMO was gel purified.
Conclusion
Navigate to the Previous or the Next Entry
 "
"