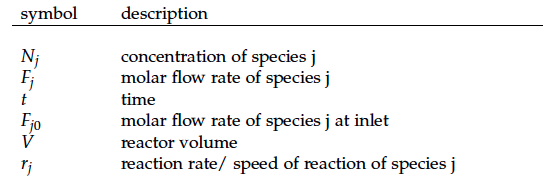
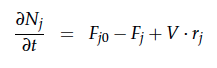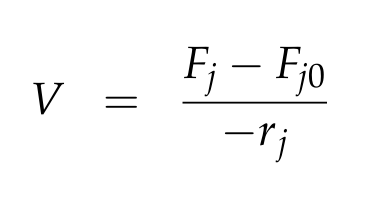Team:DTU-Denmark/Reactor Model
From 2013.igem.org
Contents |
The Reactor
Summary
We want to get a first estimate of how large a reactor filled with our transformed E. coli cells must be to treat ammonia polluted wastewater. Because Mutant 2 requires anaerobic conditions, we plan to build two reactors in series and find with the help of modeling that the second should be around 10 times the size of the first. This is under the assumption that all transformed proteins will be expressed at the same level, so expressing proteins at different levels is something we should look into if we want to avoid a large difference in reactor sizes.
Methods
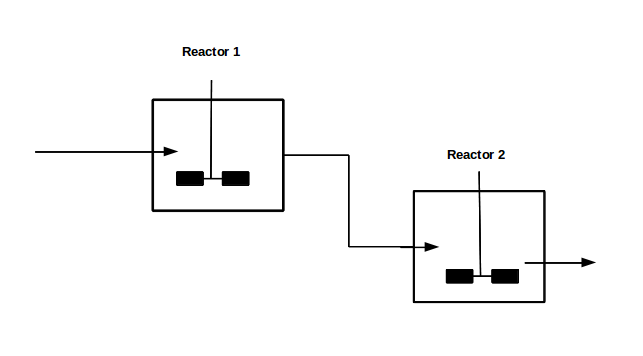
With our genetically engineered E. coli we want to treat ammonia polluted waste water. The fastest way to do this would be in a continuous process like a continuous stirred tank reactor (CSTR). Ideally this process would generate an amount of nitrous oxide that is so large that the energy needed for running the system can be delivered by catalytically decomposing the nitrous oxide. We are planing to test a simple CSTR system but hold the engineered E. coli back in the reactor, so that the outgoing water is free of bacteria. Also we will need to use two CSTRs in sequence because the second mutant requires anaerobic conditions. A picture of two CSTR reactors in series can be found in Figure 1. Another interesting solution would be to grow a bacterial biofilm on solid carriers as shown in Figure 2, a technology known as moving bed biofilm reactor.
Figure 1: Two CSTRs in series.
Figure 2: In moving bed biofilm reactors the biofilm is grown on carriers as shown above. The picture is taken from http://www.hydrotech-group.com/en/products/industrial-wwtp/mbbr/.
A CSTR can be balanced using the following equation [1]:
For a CSTR perfect mixing is assumed, which means that the concentrations of all compounds are the same everywhere in the reactor and also at the exit point. If the system is run in steady state the so called design equation can be used to calculate the necessary volume of the reactor:
We can split the molar flow rate of species j into concentration of species j times flow. The reaction rate is dependent on the substrate concentration and can be described with Michaelis-Menten kinetics in our case: ![]() . So we can calculate the volume of the first reactor for series of different flow rates and substrate concentrations.
To include the right enzyme concentration we need to estimate how many cells per
litre we will have in the reactor and how many enzymes each cell will contain. It
was estimated that the cells take up 40% of the volume in the reactor and that they
contain 1000 copies of each protein of the pathway (see kinetic modeling) resulting in a enzyme
concentration of 8.456 nmol/L.
However this equation does not allow a complete removal of the substrate ammonia
from the system, therefore we look at substrate outlet values around 10% of the
substrate inlet values (this corresponds to 90% of NH3 conversion to NO2 ).
The matlab function describing the design equation and the script to evaluate it
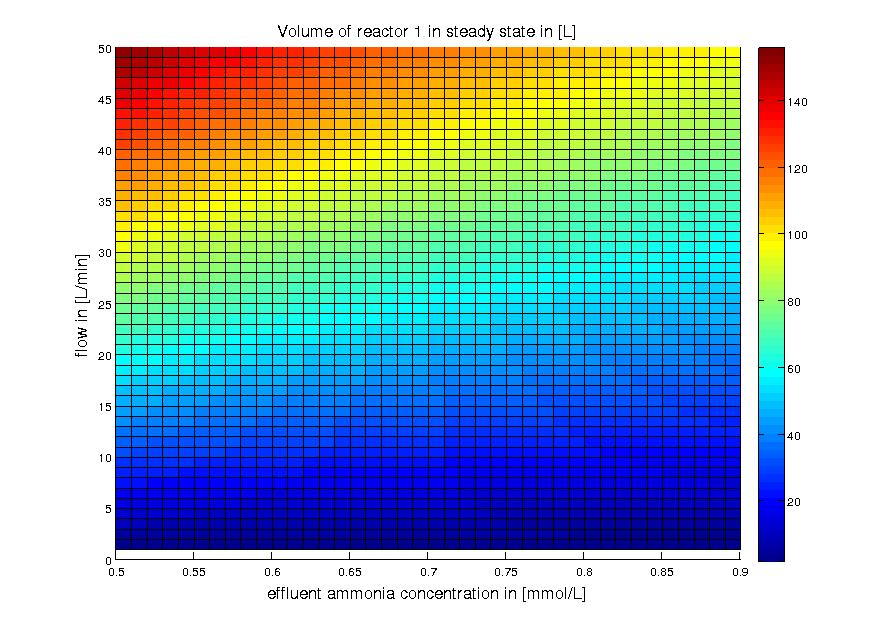
for different flows and substrate concentrations can be found under scripts below. The result of evaluating the design equation for
different flows and substrate concentrations is shown in Figure 3.
. So we can calculate the volume of the first reactor for series of different flow rates and substrate concentrations.
To include the right enzyme concentration we need to estimate how many cells per
litre we will have in the reactor and how many enzymes each cell will contain. It
was estimated that the cells take up 40% of the volume in the reactor and that they
contain 1000 copies of each protein of the pathway (see kinetic modeling) resulting in a enzyme
concentration of 8.456 nmol/L.
However this equation does not allow a complete removal of the substrate ammonia
from the system, therefore we look at substrate outlet values around 10% of the
substrate inlet values (this corresponds to 90% of NH3 conversion to NO2 ).
The matlab function describing the design equation and the script to evaluate it
for different flows and substrate concentrations can be found under scripts below. The result of evaluating the design equation for
different flows and substrate concentrations is shown in Figure 3.
The second reactor takes the effluent water of the first reactor as feed water. Assuming 90% of the NH3is converted to NO2 this means that the feed water of the second reactor will contain 5.2848 mM NO2 (if the NH3 concentration in the feed water of the first reactor was 5.872 mM as described above). Toreach an initial estimation of the necessary reactor size we assume that the conversion of NO to N2O happens instantaneously. This is reasonable because NO is toxic to the cells and should therefore not accumulate, further the reaction of NO2 to NO is slower than that of NO to N2O. Whith this assumption we can calculate the necessary volume of the second reactor in the same way as for the fist. The result is shown in Figure 4.
Results and Discussion
The required volume of reactors 1 and 2 to convert around 80% of the of the ammonia in water polluted with 100 mg/L ammonia is shown in Figures 3 and 4. The result is of course highly dependent on the desired flow. Overall reactor 2 should be around times as big as reactor 1. However the underlying assumption here is that all transformed proteins are produced at the level. Perhaps it will make sense to vary expression levels in order to reduce the size difference of the two reactors.
Figure 3:<b/> Reactor 1: volume for different flow rates and substrate (ammonia) effluent water concentrations around 10% of the substrate feed water concentrations. The ammonia concentration in the feed water is 100 mg/L (5.872 mM). The volume was calculated for a steady state application using the design equation.
References
[1] H. Scott Fogler. Essentials of Chemical Reaction Engineering. Prentice Hall, 2010.
 "
"