Team:ETH Zurich/Optimization
From 2013.igem.org
| Line 12: | Line 12: | ||
<h1> Introduction of a negative feedback loop to reduce LuxR levels </h1> | <h1> Introduction of a negative feedback loop to reduce LuxR levels </h1> | ||
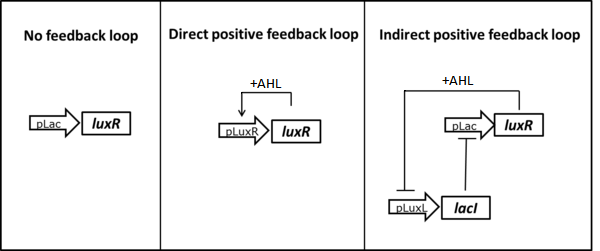
| - | As described in the [https://2013.igem.org/Team:ETH_Zurich/Circuit circuit optimization] part we tried to lower the amount of LuxR present in the uninduced state testing two different positive feedback loop motives. | + | As described in the [https://2013.igem.org/Team:ETH_Zurich/Circuit circuit optimization] part we tried to lower the amount of LuxR present in the uninduced state by testing two different positive feedback loop motives (Figure 3). The constructs were tested in liquid culture over time and compared to the basic receiver cell circuit with constitutive expression of LuxR. The experimental set-up and data analysis was done similar to the experiment described above. |
| + | |||
[[File:ETHZ feedbackstrategies.png|center|600px|thumb|<b>Figure 3: </b> Possible strategies to reduce basal LuxR expression using positive feedback loops.]] | [[File:ETHZ feedbackstrategies.png|center|600px|thumb|<b>Figure 3: </b> Possible strategies to reduce basal LuxR expression using positive feedback loops.]] | ||
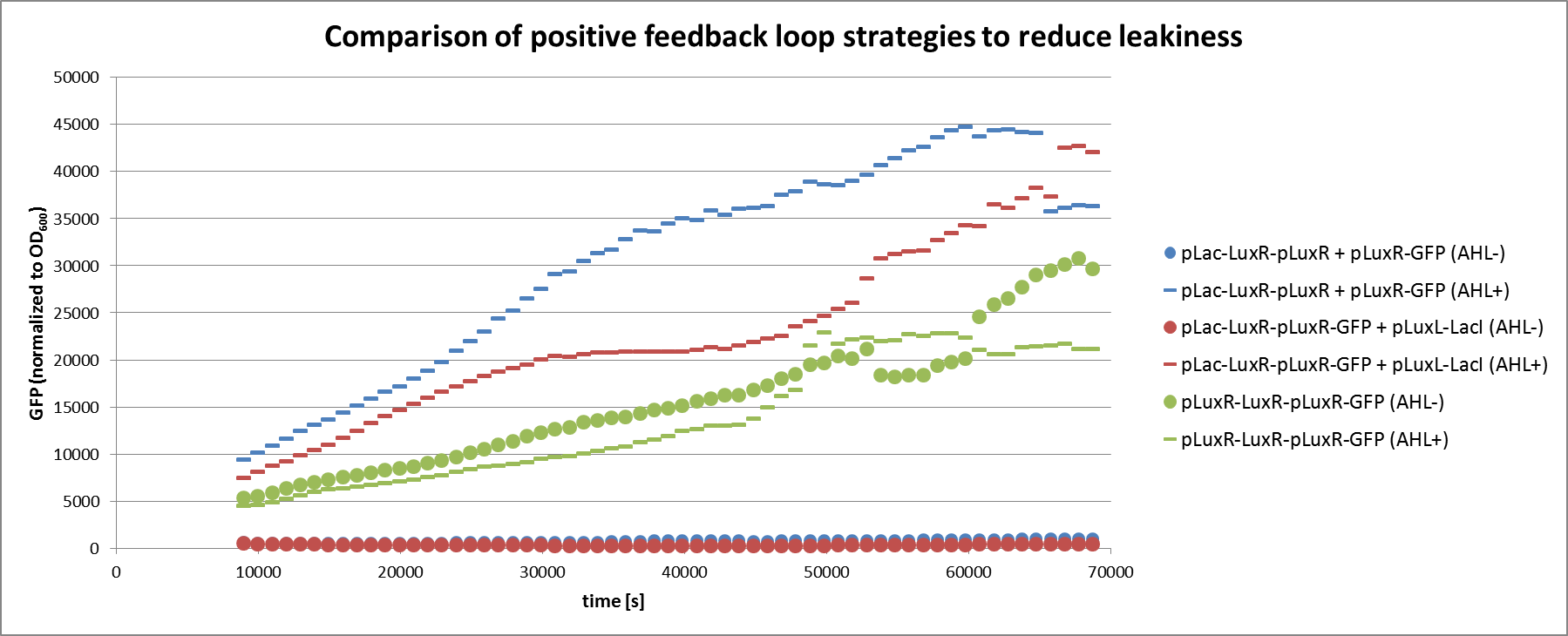
| - | [[File:ETHZ feedbackgraph.png| | + | [[File:ETHZ feedbackgraph.png|center|850px|thumb|<b>Figure 4: </b> Analysis of the non-optimized circuit and the two feedback strategies in liquid culture experiment. The circuits tested correspond to the ones described in Figure 3. All circuits were tested with or without induction through AHL.]] |
| - | [[File:ETHZ feedbackstrategiesgraphOnOff.png| | + | <br> |
| + | [[File:ETHZ feedbackstrategiesgraphOnOff.png|left|600px|thumb|<b>Figure 5: </b> Comparison of the max. observed On/Off ratios using either one of the positive feedback loop strategies. The On/Off ratios were obtained by dividing the normalized fluorescence in the induced state by the fluorescence of the uninduced circuit. The max. value reached within measuring time was taken for comparison.]] | ||
| + | |||
| + | We can clearly see from the data that the direct feedback loop were LuxR is put under its own promoter does not work (Figure 4). There is no difference visible between the induced and the uninduced state. We assume that the high plasmid copy number of the construct and the resulting high amount of LuxR could be the reason. The second strategy with the indirect feedback proved to work very well instead. <b>Comparing the maximal On/Off ratios achieved during the time course, we can see a two-fold improvement of the indirect feedback loop over the non-optimized circuit </b>(Figure 5). The reason for the high On/Off ratio is a almost complete reduction of the basal GFP expression. | ||
| + | |||
<br clear="all"/> | <br clear="all"/> | ||
Revision as of 19:46, 27 October 2013
Contents |
Evaluation of the leakiness within the receiver cell system
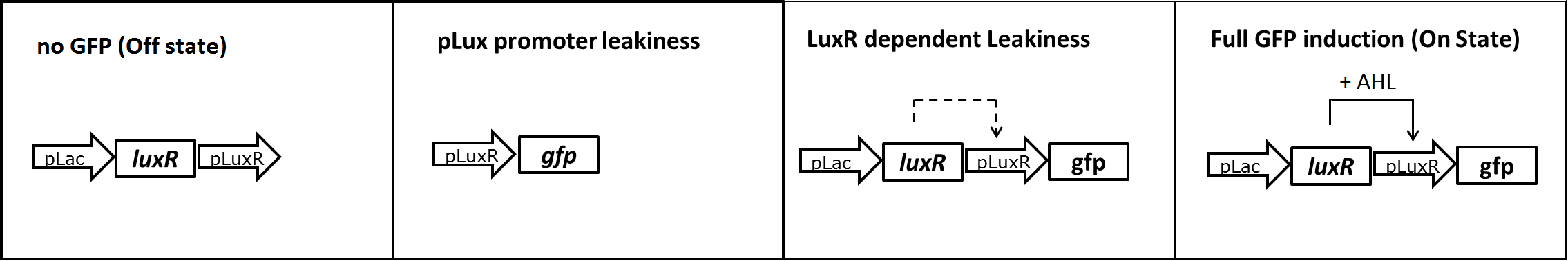
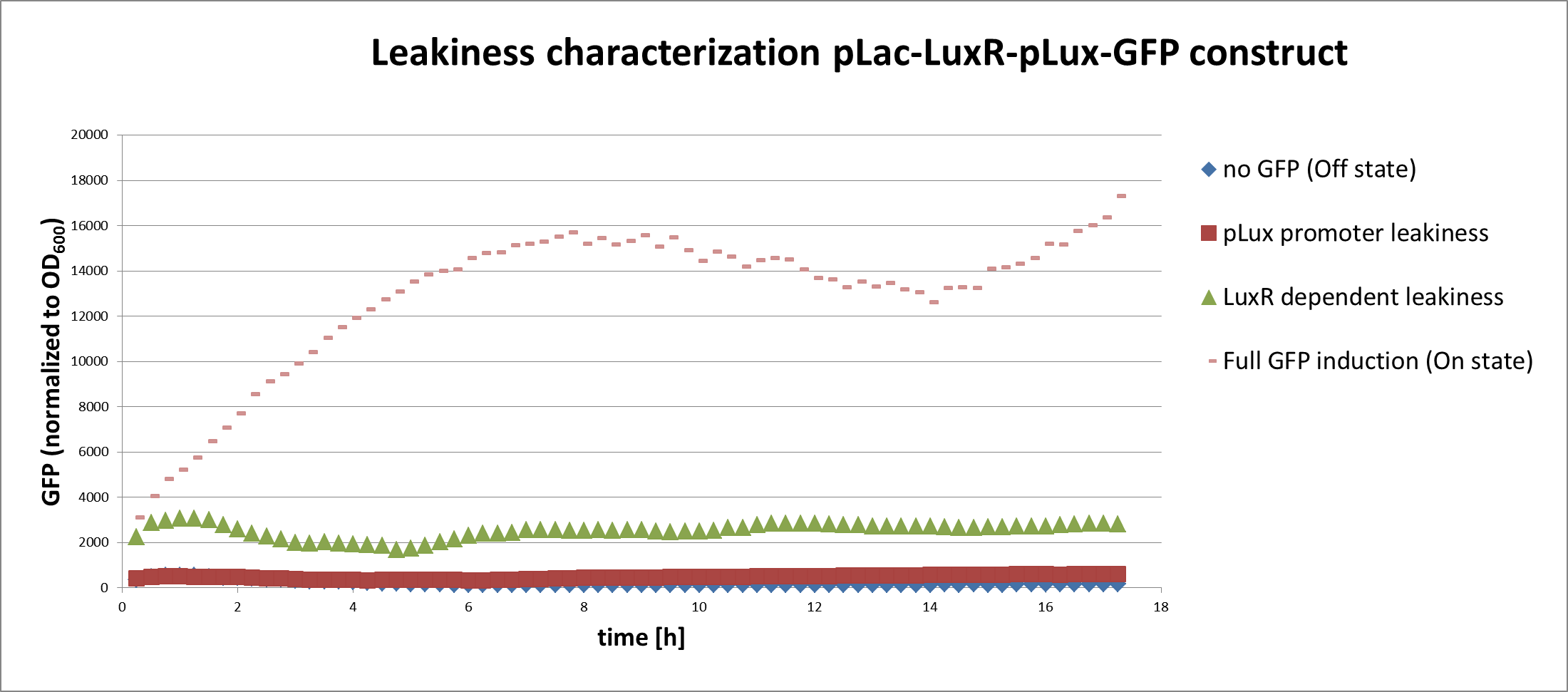
In a first experiment we had to find the source of the leakiness in our basic receiver cell system. In the absence of AHL the expression of GFP can either result from an inducer-independent activation of the pLuxR promoter or from an AHL-independent activation of the promoter through the LuxR protein. To test for these two possibilities we tested different constructs (Figure 1) in liquid cultures and analyzed the GFP fluorescence over time with the TECAN plate reader. The experiments were conducted in triplicates and for activation 100nM AHL was used. Levels of fluorescence were normalized to the OD600.
The pLac-LuxR-pLuxR-GFP reporter system showed significant leakiness in the absence of AHL. We defined the background using a construct without GFP (Figure 1, first construct). We used a simple pLuxR-GFP construct without LuxR protein (Figure 1, second construct) to measure the basal expression resulting from promoter leakiness alone. The measured signal is comparable to background levels, suggesting that pLuxR promoter per se is remarkably tight.
The complete pLac-Lux-pLuxR-GFP receiver construct (Figure 1, third construct) in the absence of AHL induction shows a jump in the measured GFP levels. This results suggest that most of the leakiness comes from an activation of the pLux promoter through LuxR alone, meaning in the absence of the AHL inducer (Figure 3).
Addition of AHL (Figure 1, fourth construct) determines a steep 5-7 times GFP induction. We concluded, that a reduction of the LuxR dependent basal activation could significantly improve the On/Off Ratio.
Introduction of a negative feedback loop to reduce LuxR levels
As described in the circuit optimization part we tried to lower the amount of LuxR present in the uninduced state by testing two different positive feedback loop motives (Figure 3). The constructs were tested in liquid culture over time and compared to the basic receiver cell circuit with constitutive expression of LuxR. The experimental set-up and data analysis was done similar to the experiment described above.
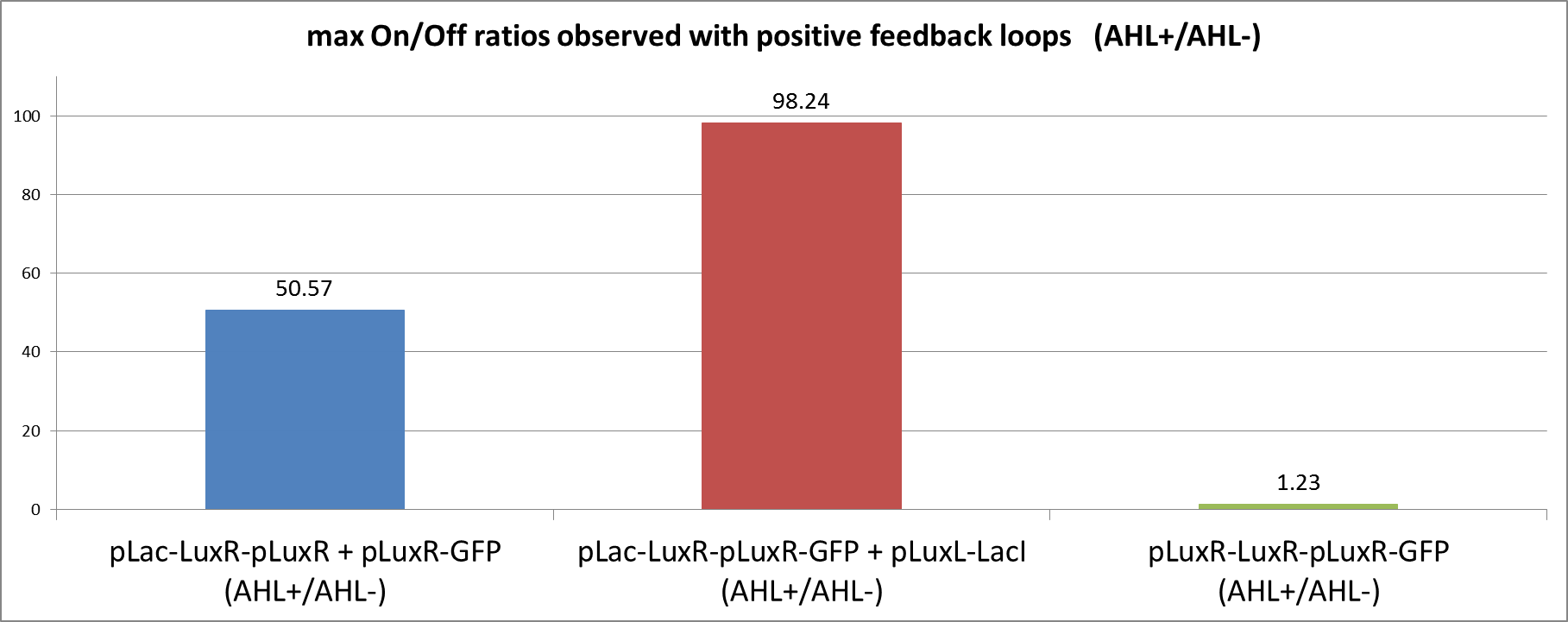
We can clearly see from the data that the direct feedback loop were LuxR is put under its own promoter does not work (Figure 4). There is no difference visible between the induced and the uninduced state. We assume that the high plasmid copy number of the construct and the resulting high amount of LuxR could be the reason. The second strategy with the indirect feedback proved to work very well instead. Comparing the maximal On/Off ratios achieved during the time course, we can see a two-fold improvement of the indirect feedback loop over the non-optimized circuit (Figure 5). The reason for the high On/Off ratio is a almost complete reduction of the basal GFP expression.
Fine-tuning of the pLac driven LuxR using glucose
Destabilization of the enzymatic reporters with degradation tags
 "
"