Team:Greensboro-Austin/MAPs
From 2013.igem.org
(→Previous work) |
(→The mussel and its glue) |
||
| Line 27: | Line 27: | ||
Mussels have an organ called the foot, which is used to both pull the mussel through terrain and to excrete a structure called the byssus. These byssal threads are made up of collagen and protein and are what allows the mussel to attach to surfaces. | Mussels have an organ called the foot, which is used to both pull the mussel through terrain and to excrete a structure called the byssus. These byssal threads are made up of collagen and protein and are what allows the mussel to attach to surfaces. | ||
| - | So why is mussel "glue" more useful over traditional glues? | + | === So why is mussel "glue" more useful over traditional glues? === |
| - | + | Mussel glue allows attachment to many different types of surfaces, both organic and inorganic. It is strong and durable, able to withstand the harsh ocean environment. It is made of protein, so it can be easily degraded and is environmentally friendly. | |
| - | + | ||
| - | + | Most importantly, however, it is biocompatible (nonimmunogenic) and—unlike most glues—it can work in aqueous environments. | |
| - | + | ||
| - | + | ||
== Mussel adhesion proteins == | == Mussel adhesion proteins == | ||
Revision as of 17:14, 17 June 2013
Contents |
The mussel and its glue
The mussel is a marine mollusk (most commonly known for its tastiness) that lives in the intertidal zone on wave washed rocks. To stand the tidal forces, they must bind to these rocks with their endogenous glue. This glue's properties make it an attractive target for synthetic improvement.
Mussels have an organ called the foot, which is used to both pull the mussel through terrain and to excrete a structure called the byssus. These byssal threads are made up of collagen and protein and are what allows the mussel to attach to surfaces.
So why is mussel "glue" more useful over traditional glues?
Mussel glue allows attachment to many different types of surfaces, both organic and inorganic. It is strong and durable, able to withstand the harsh ocean environment. It is made of protein, so it can be easily degraded and is environmentally friendly.
Most importantly, however, it is biocompatible (nonimmunogenic) and—unlike most glues—it can work in aqueous environments.
Mussel adhesion proteins
There are several different proteins that make up the byssal threads, but the ones we’re most interested in are the ones that lie at the interface with the surface to which the mussel adheres. These proteins are responsible for the binding to the substrates and therefore have the most potential for use as a glue.
Adhesion relies on post-translational modifications
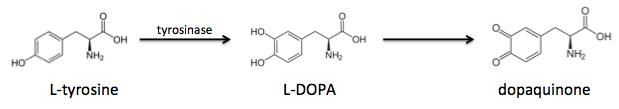
All of the different mussel foot proteins have a percentage of their tyrosines post-translationally modified to a molecule called L-DOPA. It turns out that the proteins closer to the adhesion surface have a much higher L-DOPA content, and in fact the L-DOPA content is linearly correlated with adhesiveness.
How MAPs adhere to surfaces
To the right is the sequence of a recombinant mussel adhesion protein, fp-151, that has good adhesive properties. Notably, it has high tyrosine content, denoted by the letter Y. It turns out that tyrosines are critical for adhesion, but first they must undergo tyrosinase-mediated hydroxylation to L-DOPA, which can be further oxidized to dopaquinone:
These modified amino acids contribute to adhesion via two separate mechanisms: L-DOPA interacts non-covalently with inorganic surfaces by chelating metal ions, whereas dopaquinone interacts with organic surfaces through the formation of covalent bonds.
Previous work
In 2007, a recombinant protein called fp-151 was first produced, which was notable for its lower cytotoxicity than previous recombinant foot proteins (Hwang et al., 2007). After purifying the protein, tyrosine residues were hydroxylated in vitro with tyrosinase, so the protein was already folded before this modification could occur, possibly limiting access to the tyrosines.
Choi, et al. extended this work with a coexpression system, such that the residues were modified in vivo, simultaneously along with translation. These foot proteins had better adhesive properties (Choi et al., 2012).
Also in 2012, a system to generate L-DOPA from free tyrosine was engineered (Satoh et al., 2012).
Limitations of previous work
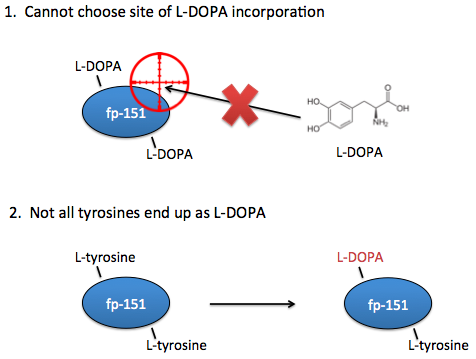
However, these systems are not yet optimal for studying the effects of L-DOPA content on adhesive properties. Ideally, you need the ability to choose exactly which sites that L-DOPA should be incorporated. In current systems, you can only choose where there should be tyrosines.
Additionally, in current systems there is no guarantee that a tyrosine placed at your desired location will even end up as an L-DOPA, so we would also want the ability to know for certain that the residues of interest are in fact L-DOPA and not tyrosine.
Our solution: direct unnatural amino acid incorportation
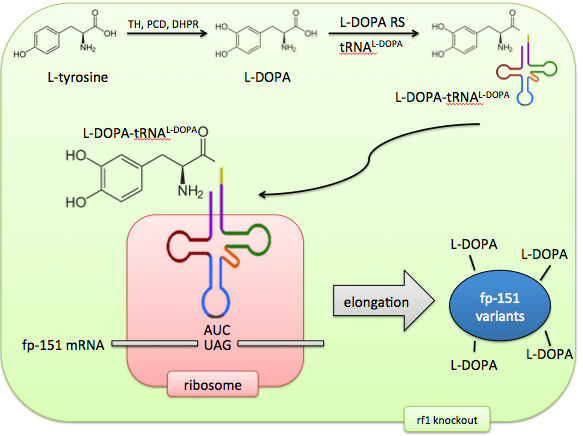
Incorporating the unnatural amino acid L-DOPA directly would solve these problems of control and predictability. Aminoacyl-tRNA synthetases, the enzymes that charge tRNAs with amino acids, can be evolved to recognize unnatural amino acids by sequential rounds of positive and negative selection. The tRNA’s anticodon loop can be engineered to recognize any codon. So, a stop codon (such as UAG, also known as the Amber codon) can be repurposed to code for a 21st amino acid.
In addition, release factor 1, which normally recognizes this Amber codon, can be knocked out, ensuring no competition between the stop codon’s old effect (termination) and its new effect (unnatural amino acid incorporation). The result of this system is an expansion of the cell’s genetic code to 21 amino acids, allowing precise control of targeted L-DOPA incorporation.
E. coli as a self-sufficient "black box"
Our vision is of E. coli as biosynthetic "factory" able to make complicated products with simple inputs. By giving it the machinery to charge tRNA with L-DOPA, and expanding the genetic code to include L-DOPA, this unnatural amino acid can be incorporated at rationally targetted locations, generating a library of foot protein variants. Appropriate with this characterization of self-sufficiency and synthetic capability, these E. coli would not even need to be supplemented with exogenous L-DOPA, as they could continually generate it from intracellular free tyrosine.
Experimental design
A two-plasmid system would be appropriate for our aims. One plasmid would code for a library of fp-151 variants, each containing Amber codons in unique locations and quantities, yielding a diverse set of foot proteins with characteristic locations and numbers of L-DOPA residues. The adhesive properties of these variants would be compared against both in vitro and in vivo tyrosinase-treated foot proteins, which would serve as positive controls.
This plasmid would also contain our L-DOPA biosynthesis cassette, ensuring a continual source of unnatural amino acid without supplementation.
The second plasmid would code for the synthetase/tRNA pair that is responsible for adding L-DOPA to the genetic vocabulary.
A side note: diversity of parts
The individual components of this system are derived from a variety of organisms. The foot proteins come from mussels, the genes in the L-DOPA biosynthesis cassette come from mice and humans, and the synthetase/tRNA pair is derived from archaea, and all these components are put in bacteria.
This covers 5 different organisms from all three domains of life, and as such would be a remarkable illustration of synthetic biology!
Project
(insert abstract here)
Make mussel adhesive proteins (MAPs) using E. coli Incorporate unnatural amino acids (How? Expand in Introduction. Stress novelty of idea.)
Natural vs. commercial underwater adhesives (Table of properties? Strengths, biocompatibility, cost, availability)
Benefits of synthetic MAPs (Why they're worth engineering)
Introduction
explain different surgical glues, downfalls of current natural ones, and emphasize potential of MAPS
Recombinant mussel adhesive proteins from Mytilus galloprovincialis
Explain properties of fp-1,2,3,4,5, dopamine, tyrosine, L-dopa, dopaquinone
explain fp 151 = fp 1 + 5 + 1
1 is a 6x repeat of 10 aa sequence
Study by (CITE SOURCE) showed that fp-151 was stickiest(?)
L-dopa can be produced through post-translational oxidation of tyrosines
---inefficient due to requirement of tyrosines from outside
Explain what RF1 does, and what suppressing it will do
-what is RF0, what strain (CITE SOURCE, give credit to lab)
-substitute ambers with some other stop codons so they still stop in RF0 strain
synthesize L-DOPA with UAAs to improve efficiency of production, and have more control on synthesis
-cell must incorporate L-dopa in tRNA/synthetase (name of plasmid, CITE SOURCE, credit the lab giving us plasmid)
Thus, AMBER codons are read as L-DOPA instead of STOP
PRIMARY GOAL: make MAPs with increased levels of L-DOPA, produced through in vivo UAA incorporation
future:
test different numbers of L-DOPAs in each of 3 geneblocks
express MAPs on cell surface, using Berkeley '09 mechanisms to get sticky microbes
or use purification techniques to extract product from the microbes
measure stickiness directly with materials techniques
measure stickiness in comparison with normal e coli (what % stick to glass in culture?)
directed evolution to obtain improved stickiness
-grow surface MAP-making e coli in flasks
---make sure e coli have the resources to mutate/incorporate L-DOPA normally
-wash the ones that didn't stick
-keep growing
-examine MAP structure after several generations
Strategy
Unnatural Amino Acid Incorporation
Previous studies have generated L-DOPA residues through post-translational modification (Choi et al. 2012, Hwang et al. 2007). However, this method has the disadvantage of less control of where L-DOPAs are incorporated and how efficient the process is. We will directly incorporate L-DOPA as an unnatural amino acid (UAA) into the MAP.
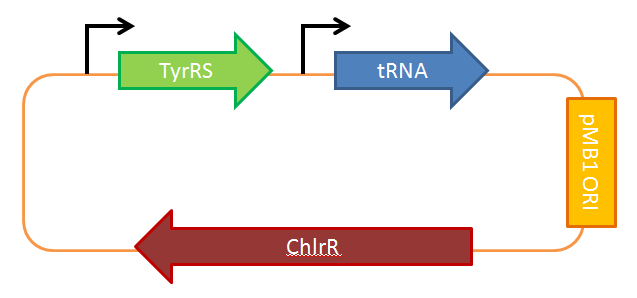
Successful UAA incorporation requires the addition of two components: a new tRNA synthetase and its corresponding tRNA. This pair must be orthogonal, meaning that they must be highly specific for each other and the UAA. The tRNA should also be complementary to the UAG stop codon (also known as the “amber” stop codon), which will be reprogrammed to code for the new UAA instead of termination.
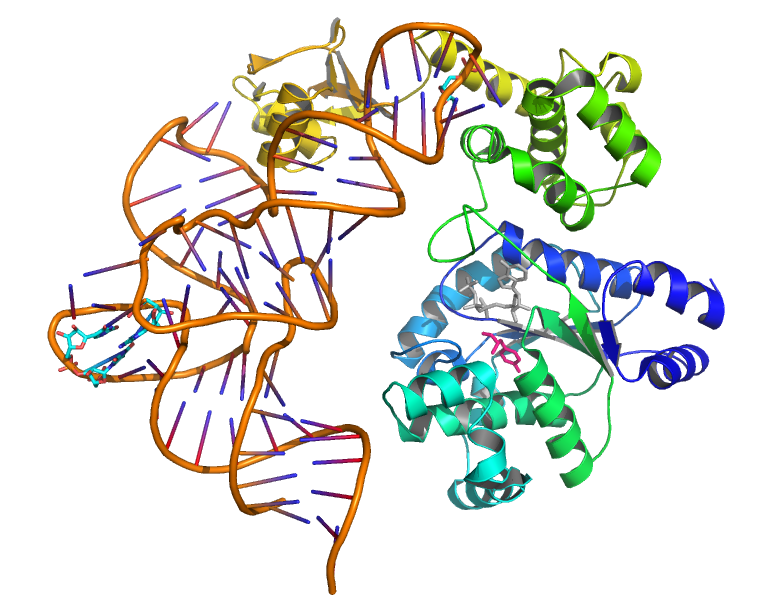
Schultz et al. have developed a system for UAA incorporation. For a starting point, they obtained tyrosyl-tRNA and tyrosyl-tRNA synthetase from Methanococcus jannaschii.This pair was chosen since the tRNA synthetase doesn’t bind to the codon binding region on the tRNA, allowing it to be reprogrammed to bind to the amber stop codon. Through a series of positive and negative selection cycles on both the tRNA and the tRNA synthetase, they were able to create a functional UAA incorporation system. We will be employing Schultz’s L-DOPA pair as shown in the circuit diagram below.
However, this pair is not perfect, since it is not completely orthogonal and will sometimes incorporate tyrosine instead of L-DOPA. For future work, it is possible to evolve a better system that is more specific.
References
Choi YS, Yang YJ, Yang B, Cha HJ. In vivo modification of tyrosine residues in recombinant mussel adhesive protein by tyrosinase co-expression in Escherichia coli. Microb Cell Fact. 2012 Oct 24;11:139. doi: 10.1186/1475-2859-11-139. PubMed PMID: 23095646; PubMed Central PMCID: PMC3533756.
Hwang DS, Gim Y, Yoo HJ, Cha HJ. Practical recombinant hybrid mussel bioadhesive fp-151. Biomaterials. 2007 Aug;28(24):3560-8. Epub 2007 May 3. PubMed PMID: 17507090.
Satoh Y, Tajima K, Munekata M, Keasling JD, Lee TS. Engineering of L-tyrosine oxidation in Escherichia coli and microbial production of hydroxytyrosol. Metab Eng. 2012 Nov;14(6):603-10. doi: 10.1016/j.ymben.2012.08.002. Epub 2012 Aug 29. PubMed PMID: 22948011.
 "
"