Team:Heidelberg/Templates/DelH week12
From 2013.igem.org
m |
|||
| Line 60: | Line 60: | ||
<br/> | <br/> | ||
[[File:Heidelberg_20130715 G1(-) G1(+) 2log G2(-) G2(+).png|200px|thumb|right|'''Fig.12.1'''gel of PCR of DelH-fragments (loaded 20 µL) <br> ''l1:''F1 without DMSO,''l2:''F1 with DMSO, ''l3:''2log ladder, ''l4:'' F2 without DMSO, ''l5:'' F2 with DMSO]] | [[File:Heidelberg_20130715 G1(-) G1(+) 2log G2(-) G2(+).png|200px|thumb|right|'''Fig.12.1'''gel of PCR of DelH-fragments (loaded 20 µL) <br> ''l1:''F1 without DMSO,''l2:''F1 with DMSO, ''l3:''2log ladder, ''l4:'' F2 without DMSO, ''l5:'' F2 with DMSO]] | ||
| - | + | ||
<br/> | <br/> | ||
No bands visible. | No bands visible. | ||
:=> Fragment 1 was not amplified. Next step is amplifying fragment 1 in two parts G1a & G1b. | :=> Fragment 1 was not amplified. Next step is amplifying fragment 1 in two parts G1a & G1b. | ||
<br/> | <br/> | ||
| + | <div style="clear:both"></div> | ||
===Amplification of DelH G1a=== | ===Amplification of DelH G1a=== | ||
====PCR Conditions G1a.W12.A==== | ====PCR Conditions G1a.W12.A==== | ||
| Line 109: | Line 110: | ||
Expected Band: 4.309 Kb | Expected Band: 4.309 Kb | ||
<br/> | <br/> | ||
| - | [[File:Heidelberg_20130716 2xG1a 4xG1b.png|200px|thumb|right|'''Fig.12. | + | [[File:Heidelberg_20130716 2xG1a 4xG1b cut.png|200px|thumb|right|'''Fig.12.3'''gel of PCR of DelH-fragments (loaded 20 µL) <br> ''l1:''F1a without DMSO,''l2:''F1a with DMSO, ''l3:''F16 without DMSO at an anealing temperature 64°C,''l5:''F16 with DMSO at an anealing temperature 64°C,''l6:''1b-57 (without DMSO), ''l7:'' 1b-57 (with DMSO),''l9'' 2 loga ladder,''l10'' Mediprep A of BB,''l11'' Mediprep B of BBF1a with DMSO]] |
| - | + | ||
| - | + | <div class="tright" style="clear:none">[[File:Heidelberg_20130716 2xG1a 4xG1b.png|200px|thumb|right|'''Fig.12.2'''gel of PCR of DelH-fragments (loaded 20 µL) <br> ''l1:''F1a without DMSO,''l2:''F1a with DMSO, ''l3:''F16 without DMSO at an anealing temperature 64°C,''l5:''F16 with DMSO at an anealing temperature 64°C,''l6:''1b-57 (without DMSO), ''l7:'' 1b-57 (with DMSO),''l9'' 2 loga ladder,''l10'' Mediprep A of BB,''l11'' Mediprep B of BBF1a with DMSO]]</div> | |
| + | |||
PCR using DMSO looks well. | PCR using DMSO looks well. | ||
:=> Fragment was cut and gel extracted. | :=> Fragment was cut and gel extracted. | ||
<br/> | <br/> | ||
| + | <div style="clear:both"></div> | ||
===Amplification of DelH G1b=== | ===Amplification of DelH G1b=== | ||
| Line 154: | Line 157: | ||
Expected Band: 4.711 Kb | Expected Band: 4.711 Kb | ||
<br/> | <br/> | ||
| - | [[File:Heidelberg_20130716 2xG1a 4xG1b.png|200px|thumb|right|'''Fig.12. | + | [[File:Heidelberg_20130716 2xG1a 4xG1b cut.png|200px|thumb|right|'''Fig.12.3'''gel of PCR of DelH-fragments (loaded 20 µL) <br> ''l1:''F1a without DMSO,''l2:''F1a with DMSO, ''l3:''F16 without DMSO at an anealing temperature 64°C,''l5:''F16 with DMSO at an anealing temperature 64°C,''l6:''1b-57 (without DMSO), ''l7:'' 1b-57 (with DMSO),''l9'' 2 loga ladder,''l10'' Mediprep A of BB,''l11'' Mediprep B of BBF1a with DMSO]] |
| - | + | <div class="tright" style="clear:none">[[File:Heidelberg_20130716 2xG1a 4xG1b.png|200px|thumb|right|'''Fig.12.2'''gel of PCR of DelH-fragments (loaded 20 µL) <br> ''l1:''F1a without DMSO,''l2:''F1a with DMSO, ''l3:''F16 without DMSO at an anealing temperature 64°C,''l5:''F16 with DMSO at an anealing temperature 64°C,''l6:''1b-57 (without DMSO), ''l7:'' 1b-57 (with DMSO),''l9'' 2 loga ladder,''l10'' Mediprep A of BB,''l11'' Mediprep B of BBF1a with DMSO]]</div> | |
| - | + | ||
| + | |||
PCR using DMSO at an annealing temperature od 57°C looks fine. | PCR using DMSO at an annealing temperature od 57°C looks fine. | ||
:=> Fragment was cut and gel extracted. | :=> Fragment was cut and gel extracted. | ||
<br/> | <br/> | ||
| - | + | <div style="clear:both"></div> | |
===Amplification of DelH G2=== | ===Amplification of DelH G2=== | ||
====PCR Conditions G2.W12.A==== | ====PCR Conditions G2.W12.A==== | ||
| Line 206: | Line 210: | ||
<br/> | <br/> | ||
[[File:Heidelberg_20130715 G1(-) G1(+) 2log G2(-) G2(+).png|200px|thumb|right|'''Fig.12.1'''gel of PCR of DelH-fragments (loaded 20 µL) <br> ''l1:''F1 without DMSO,''l2:''F1 with DMSO, ''l3:''2 log ladder,''l4:''F2 (without DMSO), ''l5:'' F2 (with DMSO)]] | [[File:Heidelberg_20130715 G1(-) G1(+) 2log G2(-) G2(+).png|200px|thumb|right|'''Fig.12.1'''gel of PCR of DelH-fragments (loaded 20 µL) <br> ''l1:''F1 without DMSO,''l2:''F1 with DMSO, ''l3:''2 log ladder,''l4:''F2 (without DMSO), ''l5:'' F2 (with DMSO)]] | ||
| - | + | ||
Expected band is visible. | Expected band is visible. | ||
:=> Fragment G2 was amplified with DMSO. Band was cut and gel extracted. | :=> Fragment G2 was amplified with DMSO. Band was cut and gel extracted. | ||
<br/> | <br/> | ||
| + | <div style="clear:both"></div> | ||
====PCR Conditions G2.W12.B==== | ====PCR Conditions G2.W12.B==== | ||
{| class="wikitable" style="float:left; margin-right:1em" | {| class="wikitable" style="float:left; margin-right:1em" | ||
| Line 254: | Line 259: | ||
<br/> | <br/> | ||
[[File:Heidelberg_20130716 M 2xG1b cut.png|200px|thumb|right|'''Fig.12.4'''gel of PCR of DelH-fragments (loaded 20 µL) <br> ''l1:''PCR of Indigoidine,''l2:''2 log ladder, ''l3-4:''G1b <br/> ''l3-4'' showed expected band at 9.6 Kb = was cut out]] | [[File:Heidelberg_20130716 M 2xG1b cut.png|200px|thumb|right|'''Fig.12.4'''gel of PCR of DelH-fragments (loaded 20 µL) <br> ''l1:''PCR of Indigoidine,''l2:''2 log ladder, ''l3-4:''G1b <br/> ''l3-4'' showed expected band at 9.6 Kb = was cut out]] | ||
| - | + | ||
Gel shows nice band at expected length. | Gel shows nice band at expected length. | ||
:=> G2 was also amplified in 50 µl and was cut and gel extracted. | :=> G2 was also amplified in 50 µl and was cut and gel extracted. | ||
<br/> | <br/> | ||
| - | + | <div style="clear:both"></div> | |
===Amplification of DelH G0=== | ===Amplification of DelH G0=== | ||
====PCR Conditions G0.W12.A==== | ====PCR Conditions G0.W12.A==== | ||
| Line 299: | Line 304: | ||
Expected band: 18.521 Kb | Expected band: 18.521 Kb | ||
<br/> | <br/> | ||
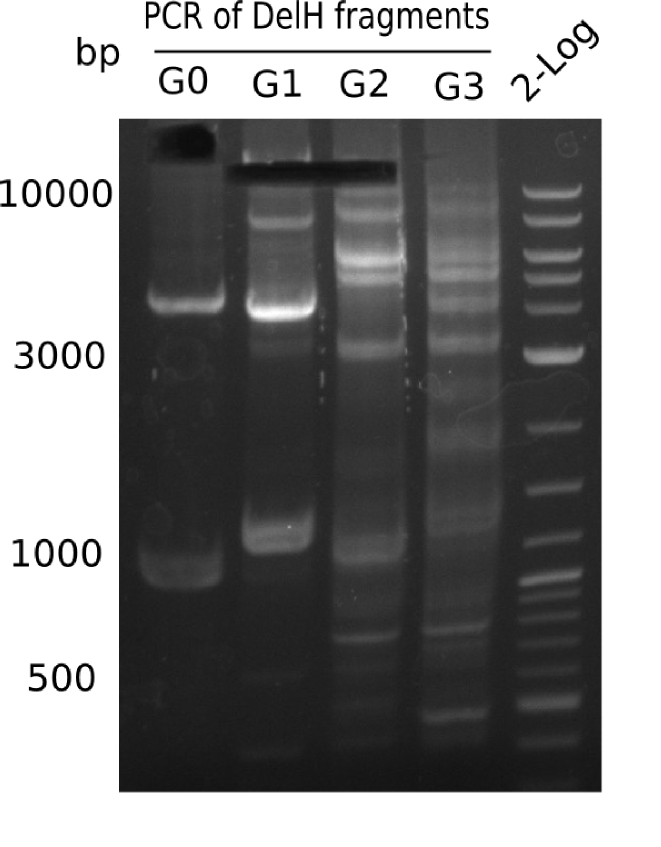
| - | [[File:Heidelberg_20130717_4xG(0-3) | + | [[File:Heidelberg_20130717_4xG(0-3)_M_cut.png |200px|thumb|right|'''Fig.12.6'''gel of PCR of DelH-fragments (loaded 20 µL) <br> ''l1-4:''DelH G0-G3 (18 Kb),''l5:''2 log ladder]] |
| - | + | <div class="tright" style="clear:none">[[File:Heidelberg_20130717_4xG(0-3)_M.png|200px|thumb|right|'''Fig.12.5'''gel of PCR of DelH-fragments (loaded 20 µL) <br> ''l1-4:''DelH G0-G3 (18 Kb),''l5:''2 log ladder]]</div> | |
| - | + | ||
| + | |||
Gel shows expected band at ~18 Kb. | Gel shows expected band at ~18 Kb. | ||
:=> Fragment G0 was cut and gel isolated. Repeat PCR to increase yield. | :=> Fragment G0 was cut and gel isolated. Repeat PCR to increase yield. | ||
| - | + | <div style="clear:both"></div> | |
====Result==== | ====Result==== | ||
Three 20 µl reactions were performed to increase yield of fragment G0. | Three 20 µl reactions were performed to increase yield of fragment G0. | ||
| Line 310: | Line 316: | ||
Expected band: 18.521 Kb | Expected band: 18.521 Kb | ||
<br/> | <br/> | ||
| - | [[File: | + | [[File:Heidelberg_20130718_3xG0_M_4xdigestedBB_M_G2b_cut.png|200px|thumb|right|'''Fig.12.8'''gel of PCR of DelH-fragments (loaded 20 µL) <br> ''l1-4:''DelH G0-G3 (18 Kb),''l5:''2 log ladder]] |
| - | + | <div class="tright" style="clear:none">[[File:Heidelberg_20130718_3xG0_M_4xdigestedBB_M_G2b.png|200px|thumb|right|'''Fig.12.7'''gel of PCR of DelH-fragments (loaded 20 µL) <br> ''l1-4:''DelH G0-G3 (18 Kb),''l5:''2 log ladder]]</div> | |
| + | |||
Gel shows expected band at ~18 Kb. | Gel shows expected band at ~18 Kb. | ||
:=> Fragment G0 was cut and gel isolated. | :=> Fragment G0 was cut and gel isolated. | ||
<br/> | <br/> | ||
| - | + | <div style="clear:both"></div> | |
===Amplification of DelH G1/2a=== | ===Amplification of DelH G1/2a=== | ||
====PCR Conditions G1/2a.W12.A==== | ====PCR Conditions G1/2a.W12.A==== | ||
| Line 356: | Line 363: | ||
Expected band: 13.083 Kb | Expected band: 13.083 Kb | ||
<br/> | <br/> | ||
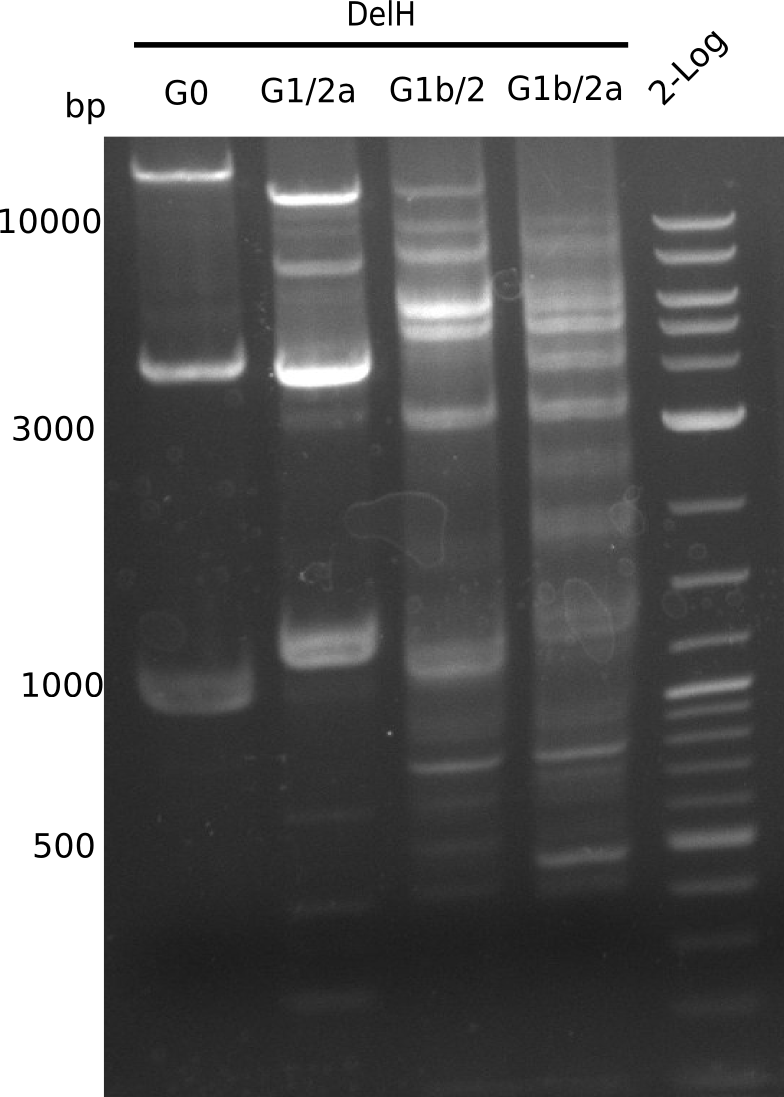
| - | [[File:Heidelberg_20130717_4xG(0-3) | + | [[File:Heidelberg_20130717_4xG(0-3)_M_cut.png |200px|thumb|right|'''Fig.12.6'''gel of PCR of DelH-fragments (loaded 20 µL) <br> ''l1-4:''DelH G0-G3 (18 Kb),''l5:''2 log ladder]] |
| - | + | <div class="tright" style="clear:none">[[File:Heidelberg_20130717_4xG(0-3)_M.png|200px|thumb|right|'''Fig.12.5'''gel of PCR of DelH-fragments (loaded 20 µL) <br> ''l1-4:''DelH G0-G3 (18 Kb),''l5:''2 log ladder]]</div> | |
| - | + | ||
| + | |||
Gel shows expected band at ~13 Kb. | Gel shows expected band at ~13 Kb. | ||
:=> Fragment G1/2a was cut and gel isolated. | :=> Fragment G1/2a was cut and gel isolated. | ||
<br/> | <br/> | ||
| + | <div style="clear:both"></div> | ||
===Amplification of DelH G1b/2=== | ===Amplification of DelH G1b/2=== | ||
====PCR Conditions G1b/2.W12.A==== | ====PCR Conditions G1b/2.W12.A==== | ||
| Line 402: | Line 411: | ||
Expected band: 14.271 Kb | Expected band: 14.271 Kb | ||
<br/> | <br/> | ||
| - | [[File:Heidelberg_20130717_4xG(0-3) | + | [[File:Heidelberg_20130717_4xG(0-3)_M_cut.png |200px|thumb|right|'''Fig.12.6'''gel of PCR of DelH-fragments (loaded 20 µL) <br> ''l1-4:''DelH G0-G3 (18 Kb),''l5:''2 log ladder]]<div class="tright" style="clear:none">[[File:Heidelberg_20130717_4xG(0-3)_M.png|200px|thumb|right|'''Fig.12.5'''gel of PCR of DelH-fragments (loaded 20 µL) <br> ''l1-4:''DelH G0-G3 (18 Kb),''l5:''2 log ladder]]</div> |
| - | + | ||
| - | + | ||
Gel shows expected band at ~14 Kb. | Gel shows expected band at ~14 Kb. | ||
:=> Fragment G1b/2 was cut and gel isolated. | :=> Fragment G1b/2 was cut and gel isolated. | ||
<br/> | <br/> | ||
| + | <div style="clear:both"></div> | ||
===Amplification of DelH G1b/2a=== | ===Amplification of DelH G1b/2a=== | ||
====PCR Conditions G1b/2a.W12.A==== | ====PCR Conditions G1b/2a.W12.A==== | ||
| Line 448: | Line 458: | ||
Expected band: 14.271 Kb | Expected band: 14.271 Kb | ||
<br/> | <br/> | ||
| - | [[File:Heidelberg_20130717_4xG(0-3) | + | [[File:Heidelberg_20130717_4xG(0-3)_M_cut.png |200px|thumb|right|'''Fig.12.6'''gel of PCR of DelH-fragments (loaded 20 µL) <br> ''l1-4:''DelH G0-G3 (18 Kb),''l5:''2 log ladder]] |
| - | + | <div class="tright" style="clear:none">[[File:Heidelberg_20130717_4xG(0-3)_M.png|200px|thumb|right|'''Fig.12.5'''gel of PCR of DelH-fragments (loaded 20 µL) <br> ''l1-4:''DelH G0-G3 (18 Kb),''l5:''2 log ladder]]</div> | |
| - | + | ||
| + | |||
Gel shows multiple bands, it is not clear, which one is correct. | Gel shows multiple bands, it is not clear, which one is correct. | ||
:=> Optimize temperatures. | :=> Optimize temperatures. | ||
<br/> | <br/> | ||
| + | <div style="clear:both"></div> | ||
===Amplification of DelH G2b=== | ===Amplification of DelH G2b=== | ||
====PCR Conditions G2b.W12.A==== | ====PCR Conditions G2b.W12.A==== | ||
| Line 494: | Line 506: | ||
Expected band: 5 Kb | Expected band: 5 Kb | ||
<br/> | <br/> | ||
| - | [[File:Heidelberg_20130718 3xG0 M 4xdigestedBB M G2b.png|200px|thumb|right|'''Fig.12. | + | [[File:Heidelberg_20130718 3xG0 M 4xdigestedBB M G2b cut.png|200px|thumb|right|'''Fig.12.8'''gel of PCR of DelH-fragments and restriction digest (loaded 20 µL) <br> ''l1-3:''DelH G0(18 Kb),''l5:''1 Kb+ ladder, ''l5:''Midiprep A digested with PstI & BamHI,''l6:''Midiprep A digested with SalI & BamHI, ''l7:''Midiprep B digested with PstI & BamHI,''l8:''Midiprep B digested with SalI & BamHI, ''l9:'' 2 log ladder, ''l10:'' fragment of DelH - G3b]] |
| - | + | <div class="tright" style="clear:none">[[File:Heidelberg_20130718 3xG0 M 4xdigestedBB M G2b.png|200px|thumb|right|'''Fig.12.7'''gel of PCR of DelH-fragments and restriction digest (loaded 20 µL) <br> ''l1-3:''DelH G0(18 Kb),''l5:''1 Kb+ ladder, ''l5:''Midiprep A digested with PstI & BamHI,''l6:''Midiprep A digested with SalI & BamHI, ''l7:''Midiprep B digested with PstI & BamHI,''l8:''Midiprep B digested with SalI & BamHI, ''l9:'' 2 log ladder, ''l10:'' fragment of DelH - G3b]]</div> | |
| + | |||
<div style="clear:both"></div> | <div style="clear:both"></div> | ||
<br/> | <br/> | ||
| Line 510: | Line 523: | ||
| Fragments present || 1 band at ~6-7 Kb || 3 bands: one bright one at ~4-5 Kb and two <br/> smaller ones at ~6 Kb and ~10 Kb || 1 band at ~6-7 Kb || 3 bands: one bright one at ~4-5 Kb and two <br/> smaller ones at ~6 Kb and ~10 Kb | | Fragments present || 1 band at ~6-7 Kb || 3 bands: one bright one at ~4-5 Kb and two <br/> smaller ones at ~6 Kb and ~10 Kb || 1 band at ~6-7 Kb || 3 bands: one bright one at ~4-5 Kb and two <br/> smaller ones at ~6 Kb and ~10 Kb | ||
|} | |} | ||
| - | [[File:Heidelberg_20130718 3xG0 M 4xdigestedBB M G2b.png|200px|thumb|right|'''Fig.12. | + | [[File:Heidelberg_20130718 3xG0 M 4xdigestedBB M G2b cut.png|200px|thumb|right|'''Fig.12.8'''gel of PCR of DelH-fragments and restriction digest (loaded 20 µL) <br> ''l1-3:''DelH G0(18 Kb),''l5:''1 Kb+ ladder, ''l5:''Midiprep A digested with PstI & BamHI,''l6:''Midiprep A digested with SalI & BamHI, ''l7:''Midiprep B digested with PstI & BamHI,''l8:''Midiprep B digested with SalI & BamHI, ''l9:'' 2 log ladder, ''l10:'' fragment of DelH - G3b]] |
| - | + | <div class="tright" style="clear:none">[[File:Heidelberg_20130718 3xG0 M 4xdigestedBB M G2b.png|200px|thumb|right|'''Fig.12.7'''gel of PCR of DelH-fragments and restriction digest (loaded 20 µL) <br> ''l1-3:''DelH G0(18 Kb),''l5:''1 Kb+ ladder, ''l5:''Midiprep A digested with PstI & BamHI,''l6:''Midiprep A digested with SalI & BamHI, ''l7:''Midiprep B digested with PstI & BamHI,''l8:''Midiprep B digested with SalI & BamHI, ''l9:'' 2 log ladder, ''l10:'' fragment of DelH - G3b]]</div> | |
| - | + | ||
| + | |||
:=> Don't trust the BB, because between 3 and 4 Kb there is no band. Backbone will be send for sequencing | :=> Don't trust the BB, because between 3 and 4 Kb there is no band. Backbone will be send for sequencing | ||
:=> Repeat entire cloning again? | :=> Repeat entire cloning again? | ||
<br/> | <br/> | ||
| - | + | <div style="clear:both"></div> | |
===Amplification of Backbone pSB6A1-lacZ-mRFP=== | ===Amplification of Backbone pSB6A1-lacZ-mRFP=== | ||
As discussed, we are going to use another backbone. It is already in the parts registry: pSB6A1 + BBa_J04450 from Spring distribution 2012 (plate 1, well K1). | As discussed, we are going to use another backbone. It is already in the parts registry: pSB6A1 + BBa_J04450 from Spring distribution 2012 (plate 1, well K1). | ||
Latest revision as of 14:01, 24 October 2013
15-07 - 21-07-13
Preparation of Primers
- All primers were 1:10 diluted
- Overview on annealing temperatures
| Primer | short2 | HM02 | HM03 | HM04 | HM05 | HM06 | HM07 | HM08 | HM09 | HM10 |
| Annealing temperature [°C] | 63 | 72.6 | 69.1 | 55.2 | 68.2 | 66.4 | 69.6 | 65.2 | 51.6 / 73.6 | 60.9 / 71.3 |
Preparation of fresh D. acidovorans
- Liquid culture: 2x 5 ml ACM medium was inocculated with picked colonies of D. acidovorans from plate (made by DN)
- Glycerol stock: was prepared from liquid culture
Amplification of DelH G1
PCR Conditions G1.W12.A
| Reagent | DelH G1 | DelH G1 |
|---|---|---|
| Template | Fresh colony of plate (by DN) | Fresh colony of plate (by DN) |
| Primer fw 10 µM | short2 | short2 |
| Primer rev 10 µM | HM04 | HM04 |
| Phusion Flash Ready Mix | 10 µl | 10 µl |
| ddH2O | 7 µl | 6 µl |
| DMSO | - | 1 µl |
| Cycles | Temperature [°C] | Time [s] |
|---|---|---|
| 1 | 98 | 5 |
| 12 | 98 | 1 |
| 63 (touchdown -0.5°C) | 5 | |
| 72 | 3:30 min | |
| 18 | 98 | 1 |
| 63 | 5 | |
| 72 | 3:30 min | |
| 1 | 72 | 10 min |
| 1 | 4 | inf |
Result
Expected band: 8.961 Kb
No bands visible.
- => Fragment 1 was not amplified. Next step is amplifying fragment 1 in two parts G1a & G1b.
Amplification of DelH G1a
PCR Conditions G1a.W12.A
| Reagent | DelH G1a | DelH G1a |
|---|---|---|
| Template | Fresh colony of plate (by DN) | Fresh colony of plate (by DN) |
| Primer fw 10 µM | short2 | short2 |
| Primer rev 10 µM | HM02 | HM02 |
| Phusion Flash Ready Mix | 10 µl | 10 µl |
| ddH2O | 7 µl | 6 µl |
| DMSO | - | 1 µl |
| Cycles | Temperature [°C] | Time [s] |
|---|---|---|
| 1 | 98 | 5 |
| 12 | 98 | 1 |
| 63 (touchdown -0.5°C) | 5 | |
| 72 | 3:30 min | |
| 18 | 98 | 1 |
| 64 | 5 | |
| 72 | 3:30 min | |
| 1 | 72 | 10 min |
| 1 | 4 | inf |
Result
Expected Band: 4.309 Kb
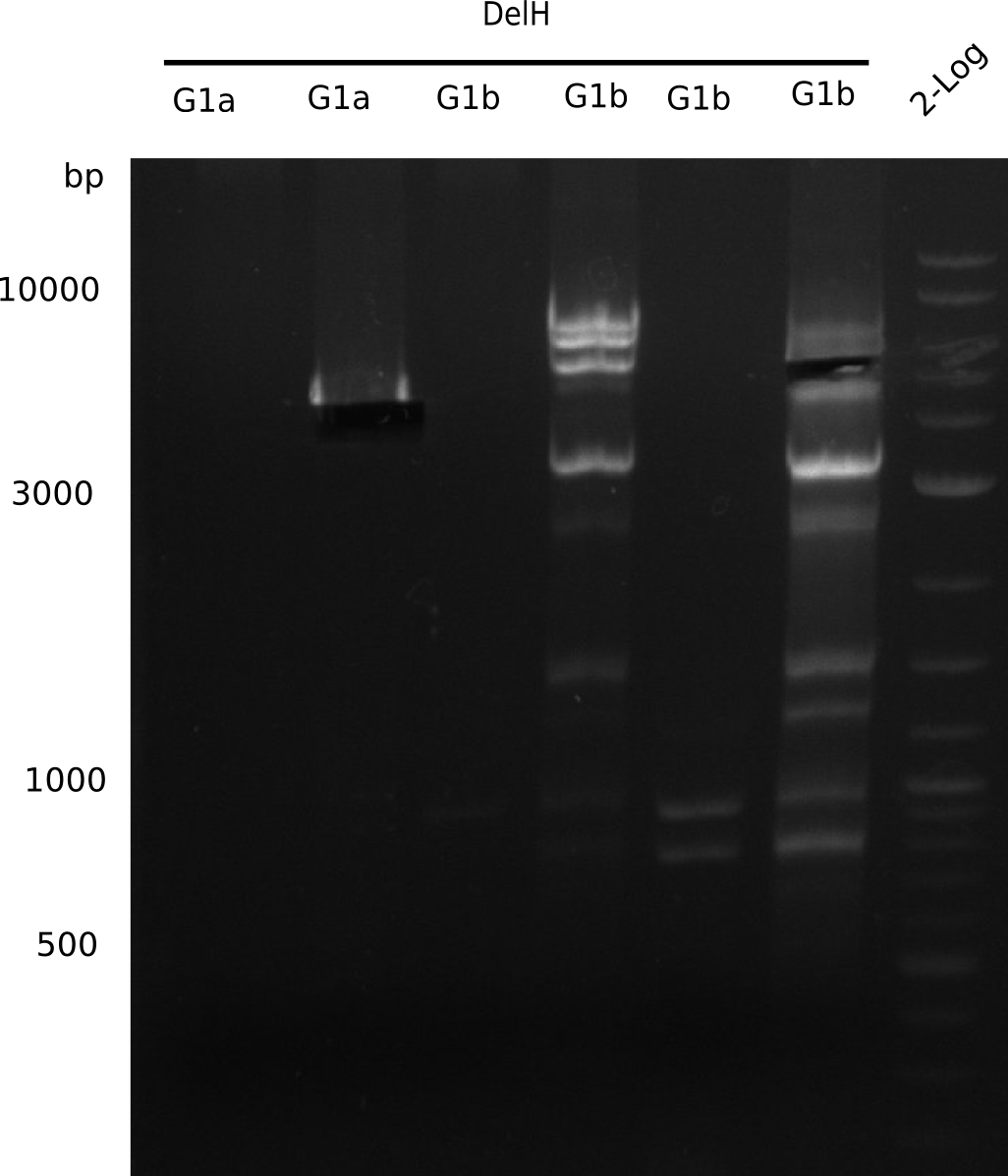
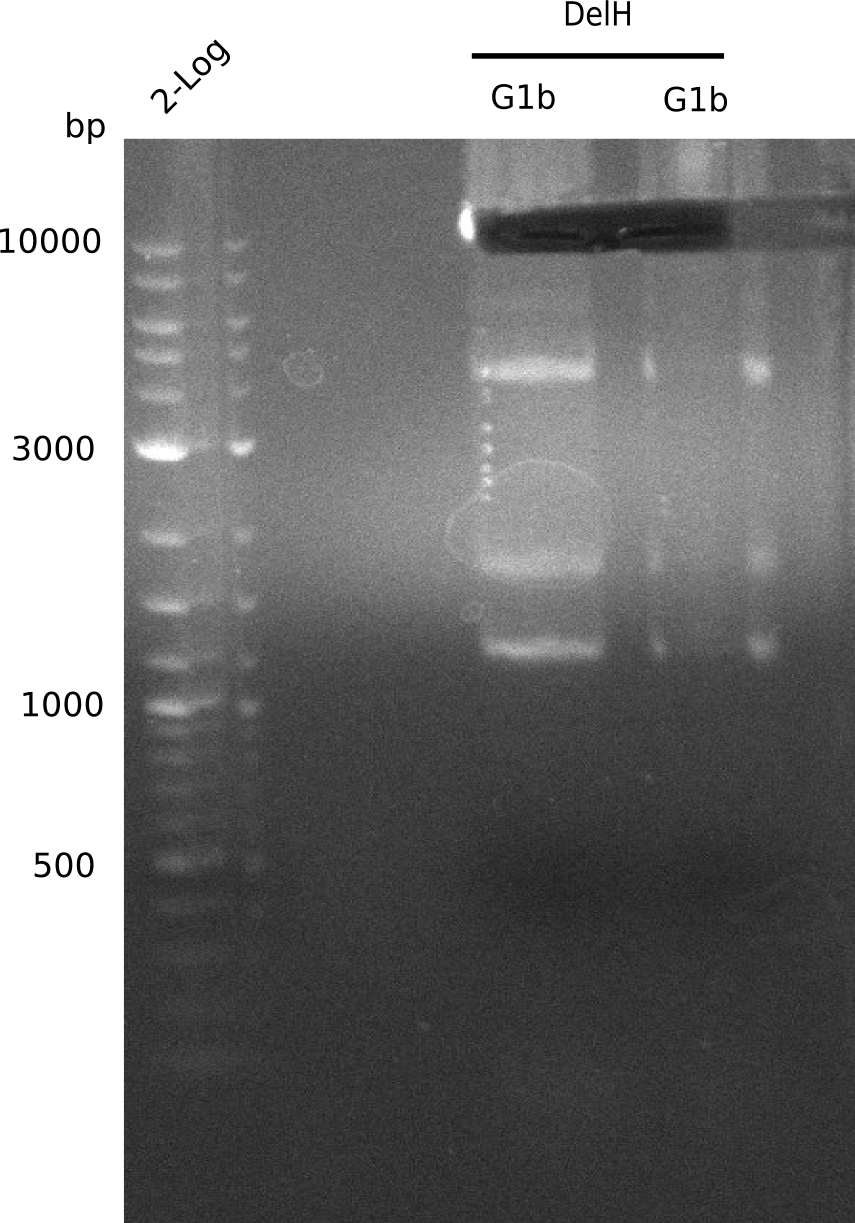
l1:F1a without DMSO,l2:F1a with DMSO, l3:F16 without DMSO at an anealing temperature 64°C,l5:F16 with DMSO at an anealing temperature 64°C,l6:1b-57 (without DMSO), l7: 1b-57 (with DMSO),l9 2 loga ladder,l10 Mediprep A of BB,l11 Mediprep B of BBF1a with DMSO
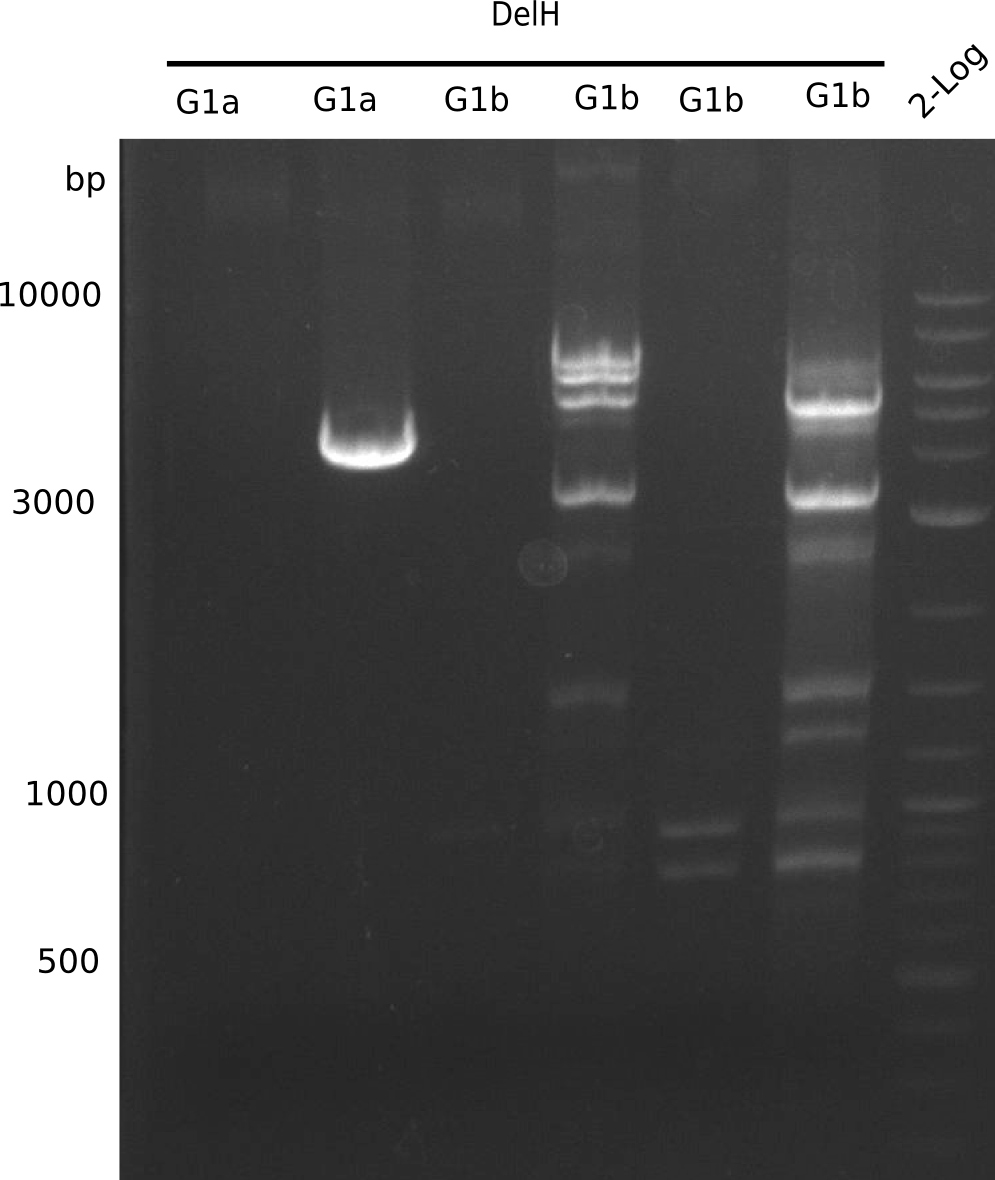
l1:F1a without DMSO,l2:F1a with DMSO, l3:F16 without DMSO at an anealing temperature 64°C,l5:F16 with DMSO at an anealing temperature 64°C,l6:1b-57 (without DMSO), l7: 1b-57 (with DMSO),l9 2 loga ladder,l10 Mediprep A of BB,l11 Mediprep B of BBF1a with DMSO
PCR using DMSO looks well.
- => Fragment was cut and gel extracted.
Amplification of DelH G1b
PCR Conditions G1b.W12.A and B
| Reagent | DelH G1b | DelH G1b |
|---|---|---|
| Template | Fresh colony of plate (by DN) | Fresh colony of plate (by DN) |
| Primer fw 10 µM | HM03 | HM03 |
| Primer rev 10 µM | HM04 | HM04 |
| Phusion Flash Ready Mix | 10 µl | 10 µl |
| ddH2O | 7 µl | 6 µl |
| DMSO | - | 1 µl |
| Cycles | Temperature A [°C] | Time [s] | Cycles | Temperature B [°C] | Time [s] | |
|---|---|---|---|---|---|---|
| 1 | 98 | 5 | 1 | 98 | 5 | |
| 30 | 98 | 1 | 30 | 98 | 1 | |
| 64 | 5 | 57 | 5 | |||
| 72 | 2:15 min | 72 | 2:15 min | |||
| 1 | 72 | 7 min | 1 | 72 | 7 min | |
| 1 | 4 | inf | 1 | 4 | inf |
Result
Expected Band: 4.711 Kb

l1:F1a without DMSO,l2:F1a with DMSO, l3:F16 without DMSO at an anealing temperature 64°C,l5:F16 with DMSO at an anealing temperature 64°C,l6:1b-57 (without DMSO), l7: 1b-57 (with DMSO),l9 2 loga ladder,l10 Mediprep A of BB,l11 Mediprep B of BBF1a with DMSO

l1:F1a without DMSO,l2:F1a with DMSO, l3:F16 without DMSO at an anealing temperature 64°C,l5:F16 with DMSO at an anealing temperature 64°C,l6:1b-57 (without DMSO), l7: 1b-57 (with DMSO),l9 2 loga ladder,l10 Mediprep A of BB,l11 Mediprep B of BBF1a with DMSO
PCR using DMSO at an annealing temperature od 57°C looks fine.
- => Fragment was cut and gel extracted.
Amplification of DelH G2
PCR Conditions G2.W12.A
| Reagent | DelH G2 | DelH G2 |
|---|---|---|
| Template | Fresh colony of plate (by DN) | Fresh colony of plate (by DN) |
| Primer fw 10 µM | 2 µl HM05 | 2 µl HM05 |
| Primer rev 10 µM | 2 µl HM08 | 2 µl HM08 |
| Phusion Flash Ready Mix | 10 µl | 10 µl |
| ddH2O | 8 µl | 7 µl |
| DMSO | - | 1 µl |
| Cycles | Temperature [°C] | Time [s] |
|---|---|---|
| 1 | 98 | 5 |
| 12 | 98 | 1 |
| 68 (touchdown -0.5°C) | 5 | |
| 72 | 3:30 min | |
| 18 | 98 | 1 |
| 67 | 5 | |
| 72 | 3:30 min | |
| 1 | 72 | 10 min |
| 1 | 4 | inf |
Result
Expected band: 9.611 Kb
Expected band is visible.
- => Fragment G2 was amplified with DMSO. Band was cut and gel extracted.
PCR Conditions G2.W12.B
| Reagent | DelH G2 |
|---|---|
| Template | Fresh colony of plate (by DN) |
| Primer fw 10 µM | 2.5 µl HM05 |
| Primer rev 10 µM | 2.5 µl HM08 |
| Phusion Flash Ready Mix | 25 µl |
| ddH2O | 17.5 µl |
| DMSO | 2.5 µl |
| Cycles | Temperature [°C] | Time [s] |
|---|---|---|
| 1 | 98 | 5 |
| 12 | 98 | 1 |
| 68 (touchdown -0.5°C) | 5 | |
| 72 | 3:30 min | |
| 18 | 98 | 1 |
| 67 | 5 | |
| 72 | 3:30 min | |
| 1 | 72 | 10 min |
| 1 | 4 | inf |
Result
Expected band: 9.611 Kb
Gel shows nice band at expected length.
- => G2 was also amplified in 50 µl and was cut and gel extracted.
Amplification of DelH G0
PCR Conditions G0.W12.A
| Reagent | DelH G0 |
|---|---|
| Template | 1 µl of glycerol stock |
| Expected length [Kb] | 18.521 |
| Primer fw 10 µM | 2.5 µl short2 |
| Primer rev 10 µM | 2.5 µl HM08 |
| Phusion Flash Ready Mix | 10 µl |
| ddH2O | 5 µl |
| DMSO | 1 µl |
| Cycles | Temperature [°C] | Time [s] |
|---|---|---|
| 1 | 98 | 5 |
| 30 | 98 | 1 |
| 65 | 5 | |
| 72 | 3:30 min | |
| 1 | 72 | 10 min |
| 1 | 4 | inf |
Result
Expected band: 18.521 Kb
Gel shows expected band at ~18 Kb.
- => Fragment G0 was cut and gel isolated. Repeat PCR to increase yield.
Result
Three 20 µl reactions were performed to increase yield of fragment G0.
Expected band: 18.521 Kb
Gel shows expected band at ~18 Kb.
- => Fragment G0 was cut and gel isolated.
Amplification of DelH G1/2a
PCR Conditions G1/2a.W12.A
| Reagent | DelH G1/2a |
|---|---|
| Template | 1 µl of glycerol stock |
| Expected length [Kb] | 13.083 |
| Primer fw 10 µM | 2.5 µl short2 |
| Primer rev 10 µM | 2.5 µl HM06 |
| Phusion Flash Ready Mix | 10 µl |
| ddH2O | 5 µl |
| DMSO | 1 µl |
| Cycles | Temperature [°C] | Time [s] |
|---|---|---|
| 1 | 98 | 5 |
| 30 | 98 | 1 |
| 65 | 5 | |
| 72 | 4:45 min | |
| 1 | 72 | 10 min |
| 1 | 4 | inf |
Result
Expected band: 13.083 Kb
Gel shows expected band at ~13 Kb.
- => Fragment G1/2a was cut and gel isolated.
Amplification of DelH G1b/2
PCR Conditions G1b/2.W12.A
| Reagent | DelH G1b/2 |
|---|---|
| Template | 1 µl of glycerol stock |
| Expected length [Kb] | 14.271 |
| Primer fw 10 µM | 2.5 µl HM03 |
| Primer rev 10 µM | 2.5 µl HM08 |
| Phusion Flash Ready Mix | 10 µl |
| ddH2O | 5 µl |
| DMSO | 1 µl |
| Cycles | Temperature [°C] | Time [s] |
|---|---|---|
| 1 | 98 | 5 |
| 30 | 98 | 1 |
| 65 | 5 | |
| 72 | 3:30 min | |
| 1 | 72 | 10 min |
| 1 | 4 | inf |
Result
Expected band: 14.271 Kb
Gel shows expected band at ~14 Kb.
- => Fragment G1b/2 was cut and gel isolated.
Amplification of DelH G1b/2a
PCR Conditions G1b/2a.W12.A
| Reagent | DelH G1b/2a |
|---|---|
| Template | 1 µl of glycerol stock |
| Expected length [Kb] | 14.271 |
| Primer fw 10 µM | 2.5 µl HM03 |
| Primer rev 10 µM | 2.5 µl HM06 |
| Phusion Flash Ready Mix | 10 µl |
| ddH2O | 5 µl |
| DMSO | 1 µl |
| Cycles | Temperature [°C] | Time [s] |
|---|---|---|
| 1 | 98 | 5 |
| 30 | 98 | 1 |
| 65 | 5 | |
| 72 | 3:30 min | |
| 1 | 72 | 10 min |
| 1 | 4 | inf |
Result
Expected band: 14.271 Kb
Gel shows multiple bands, it is not clear, which one is correct.
- => Optimize temperatures.
Amplification of DelH G2b
PCR Conditions G2b.W12.A
| Reagent | DelH G2b |
|---|---|
| Template | 1 µl of glycerol stock |
| Expected length [Kb] | 5 |
| Primer fw 10 µM | 2.5 µl HM07 |
| Primer rev 10 µM | 2.5 µl HM08 |
| Phusion Flash Ready Mix | 10 µl |
| ddH2O | 5 µl |
| DMSO | 1 µl |
| Cycles | Temperature [°C] | Time [s] |
|---|---|---|
| 1 | 98 | 5 |
| 30 | 98 | 1 |
| 68 | 5 | |
| 72 | 2:00 min | |
| 1 | 72 | 5 min |
| 1 | 4 | inf |
Result
Expected band: 5 Kb
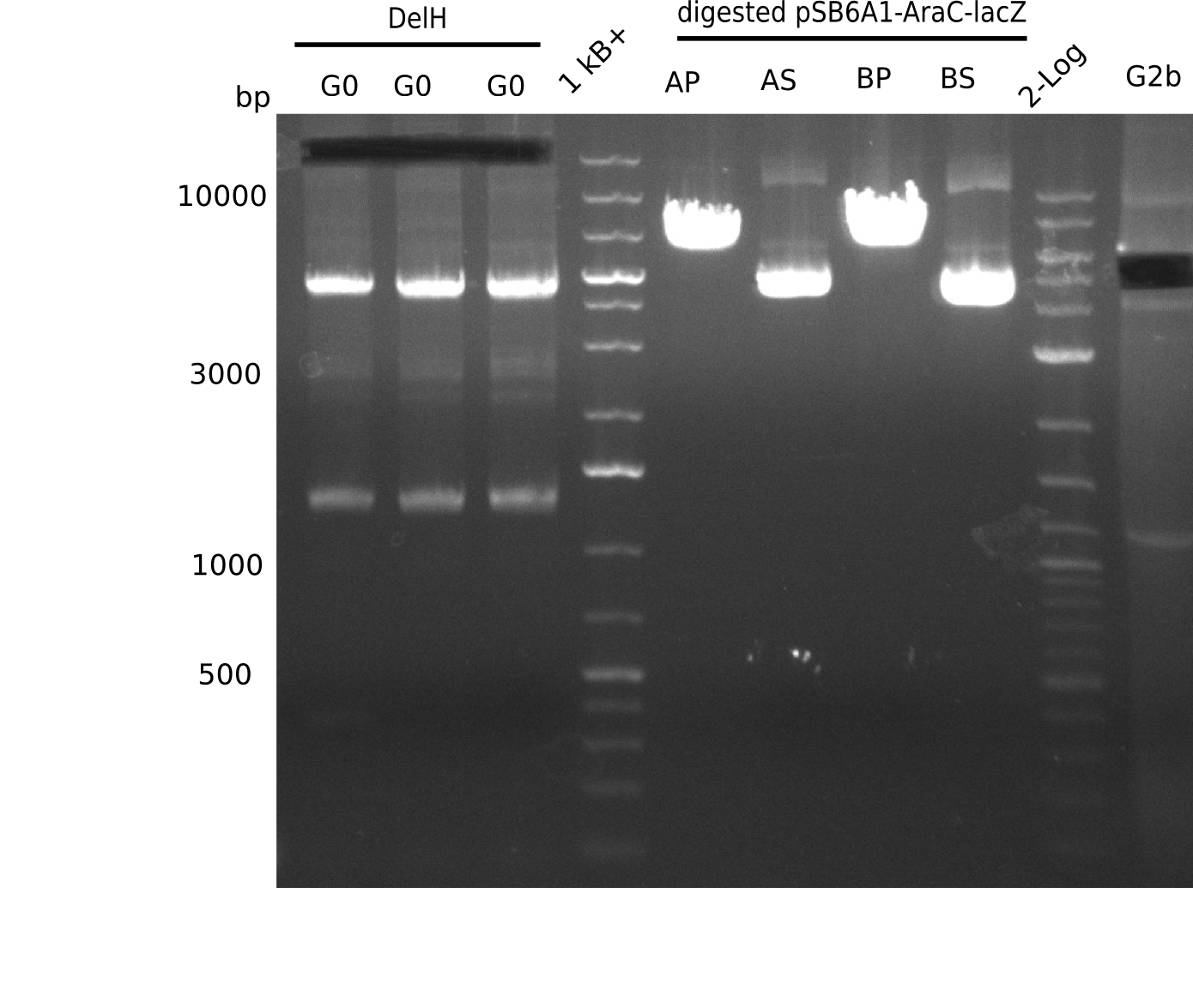
l1-3:DelH G0(18 Kb),l5:1 Kb+ ladder, l5:Midiprep A digested with PstI & BamHI,l6:Midiprep A digested with SalI & BamHI, l7:Midiprep B digested with PstI & BamHI,l8:Midiprep B digested with SalI & BamHI, l9: 2 log ladder, l10: fragment of DelH - G3b
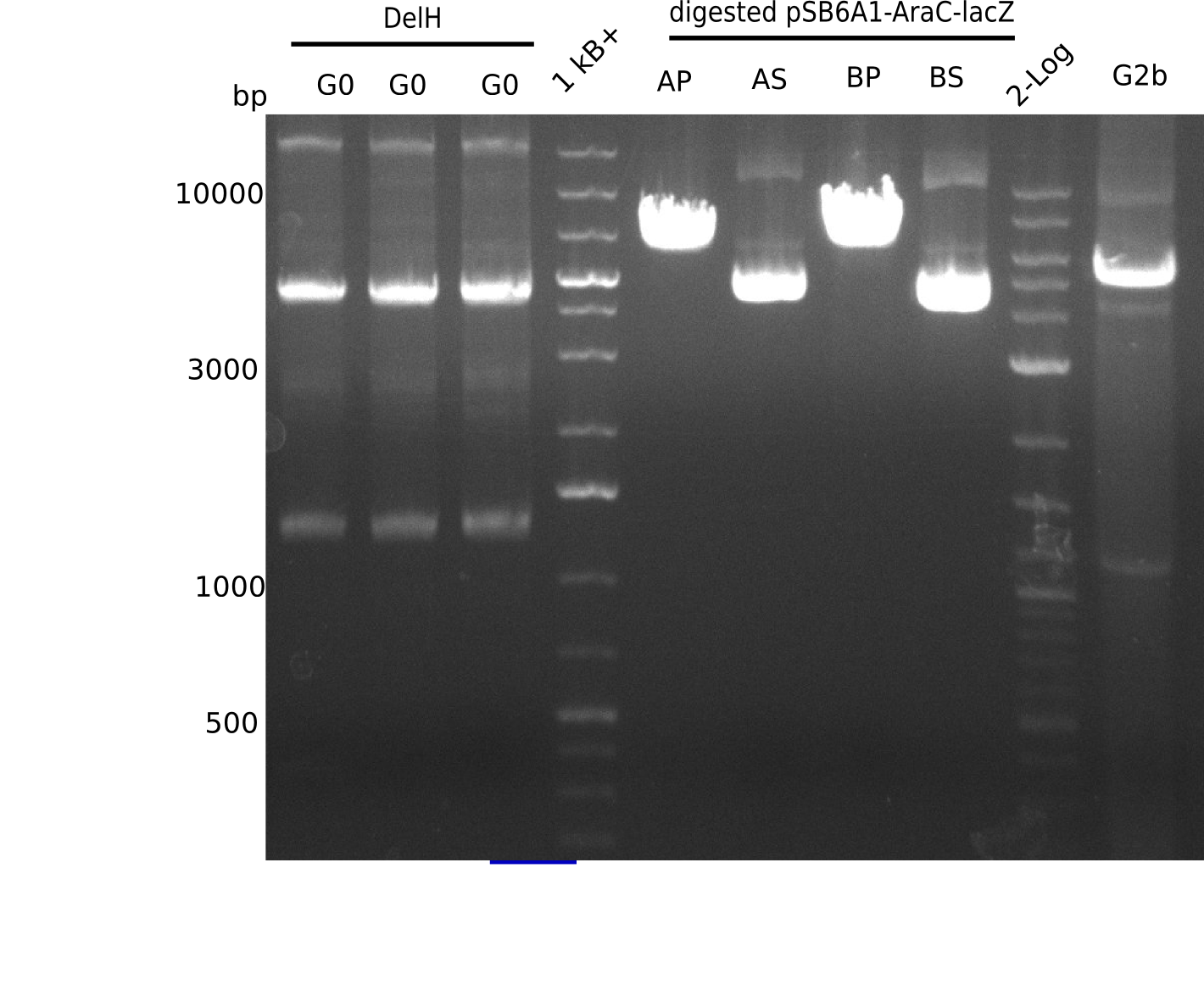
l1-3:DelH G0(18 Kb),l5:1 Kb+ ladder, l5:Midiprep A digested with PstI & BamHI,l6:Midiprep A digested with SalI & BamHI, l7:Midiprep B digested with PstI & BamHI,l8:Midiprep B digested with SalI & BamHI, l9: 2 log ladder, l10: fragment of DelH - G3b
Amplification of Backbone pSB6A1-AraC-lacZ
Test Restriction Digest of Midiprep
| Midiprep | A | A | B | B |
|---|---|---|---|---|
| Enzymes | BamHI & Pst | BamHI & SalI | BamHI & PstI | BamHI & SalI |
| Wanted fragments [Kb] | 3.317 & 4.071 | 3.794 & 3.594 | 3.317 & 4.071 | 3.794 & 3.594 |
| Fragments present | 1 band at ~6-7 Kb | 3 bands: one bright one at ~4-5 Kb and two smaller ones at ~6 Kb and ~10 Kb | 1 band at ~6-7 Kb | 3 bands: one bright one at ~4-5 Kb and two smaller ones at ~6 Kb and ~10 Kb |

l1-3:DelH G0(18 Kb),l5:1 Kb+ ladder, l5:Midiprep A digested with PstI & BamHI,l6:Midiprep A digested with SalI & BamHI, l7:Midiprep B digested with PstI & BamHI,l8:Midiprep B digested with SalI & BamHI, l9: 2 log ladder, l10: fragment of DelH - G3b

l1-3:DelH G0(18 Kb),l5:1 Kb+ ladder, l5:Midiprep A digested with PstI & BamHI,l6:Midiprep A digested with SalI & BamHI, l7:Midiprep B digested with PstI & BamHI,l8:Midiprep B digested with SalI & BamHI, l9: 2 log ladder, l10: fragment of DelH - G3b
- => Don't trust the BB, because between 3 and 4 Kb there is no band. Backbone will be send for sequencing
- => Repeat entire cloning again?
Amplification of Backbone pSB6A1-lacZ-mRFP
As discussed, we are going to use another backbone. It is already in the parts registry: pSB6A1 + BBa_J04450 from Spring distribution 2012 (plate 1, well K1).
Transformation in E. coli TOP10
- Chemical transformation of 3 µl of the plasmid
- Incubation on ice, 15 min
- Heat shock 42°C, 40 s
- Incubation on ice again
- Resuspended in 500 µl LB Amp for 30 min
- Centrifuged (120, 5,000 rpm), removal of supernatant
- Plated on LB Amp plate
- Incubation ON at 37°C
 "
"