Team:Heidelberg/Templates/DelH week21
From 2013.igem.org
(Created page with " == 16-09 - 22-09-13 == ===Generation of DelH Plasmid pHM04 15-09=== ====Colony-PCR Conditions CP.W21.A==== {| class="wikitable" style="float:left; margin-right:1em" |- | Templat...") |
|||
| Line 1: | Line 1: | ||
| - | == 16-09 - 22-09-13 == | + | ==16-09 - 22-09-13 == |
===Generation of DelH Plasmid pHM04 15-09=== | ===Generation of DelH Plasmid pHM04 15-09=== | ||
====Colony-PCR Conditions CP.W21.A==== | ====Colony-PCR Conditions CP.W21.A==== | ||
| Line 43: | Line 43: | ||
Expected band: 663 bp | Expected band: 663 bp | ||
<br/> | <br/> | ||
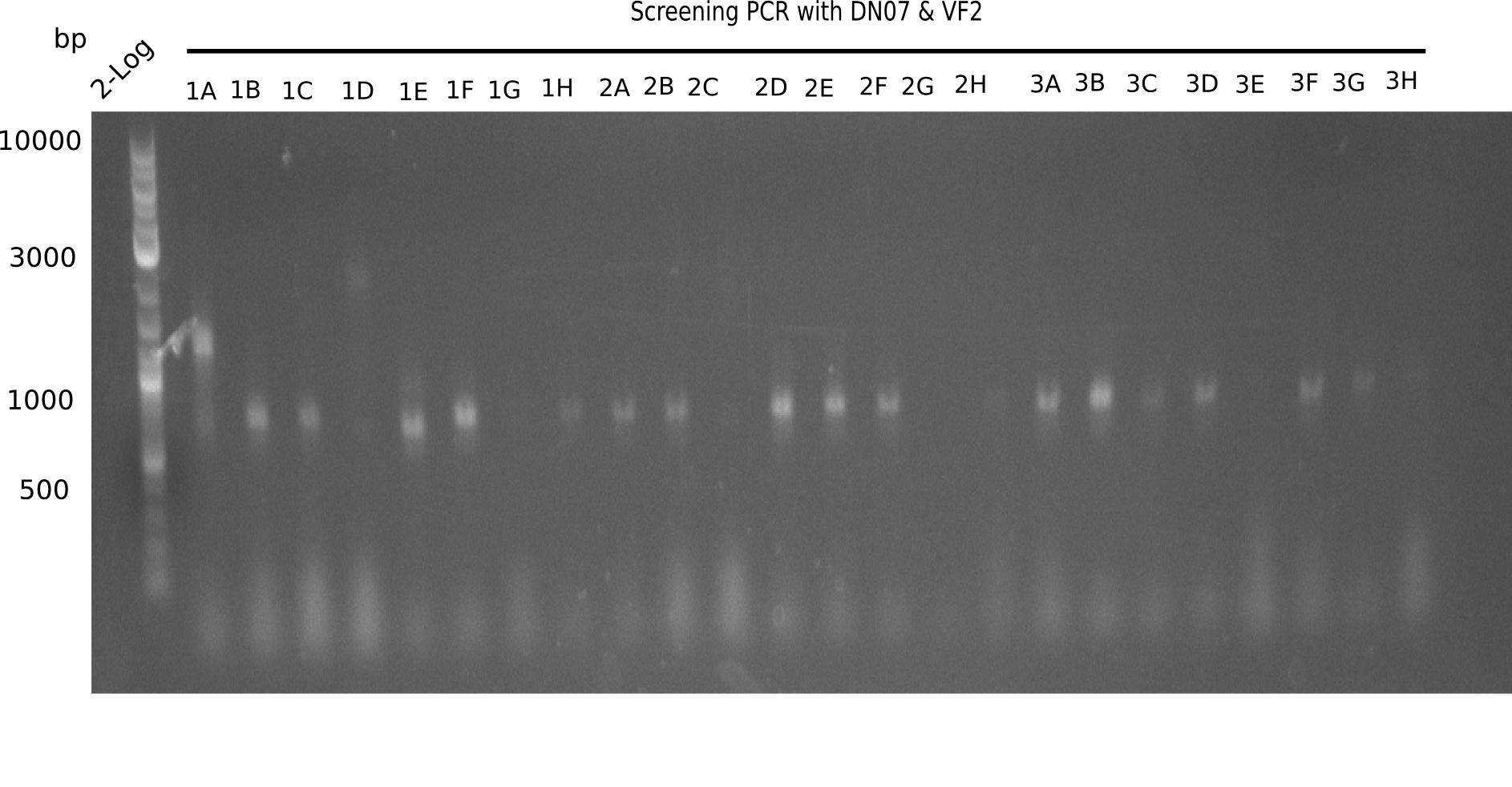
| - | [[File: | + | [[File:Heidelberg_20130916 2log Screen 1-3.png|200px|thumb|right|'''Fig.21.1''' Colony PCR of Gibson assembled DelH-BB without mRFP 96 well plate I(loaded 10 µL of PCR) <br> ''l1:''50 bp ladder, ''l2-l26:''colonies 1A-4A ]] |
| - | [[File: | + | [[File:Heidelberg_20130916 2log Screen 4-6.png|200px|thumb|right|'''Fig.21.2''' Colony PCR of Gibson assembled DelH-BB without mRFP 96 well plate I (loaded 10 µL of PCR) <br> ''l1:''50 bp ladder, ''l2-l26:''colonies 4B-7B ]] |
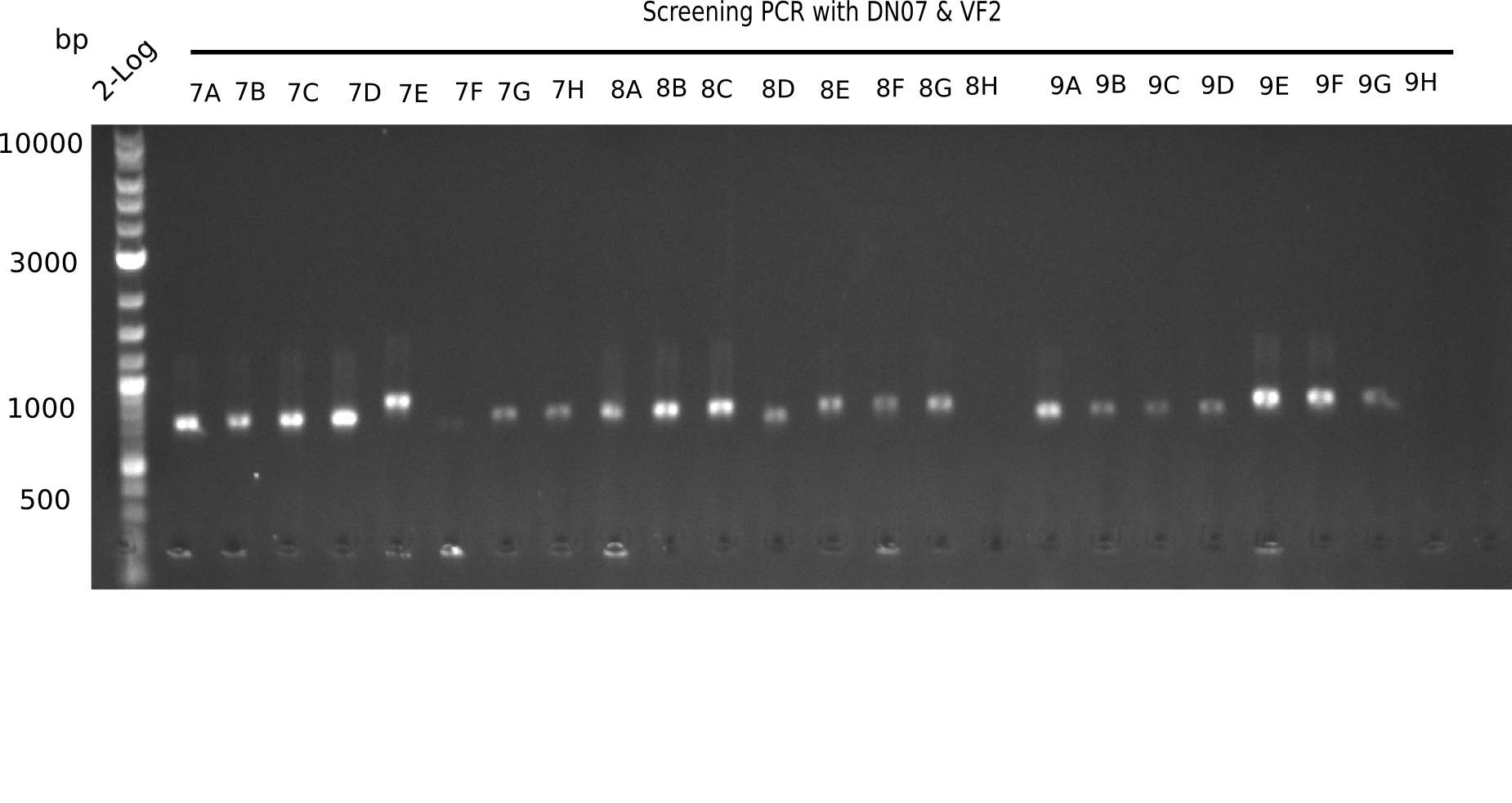
| - | [[File: | + | [[File:Heidelberg_20130916 2log Screen 7-9.png|200px|thumb|right|'''Fig.21.3''' Colony PCR of Gibson assembled DelH-BB without mRFP 96 well plate I(loaded 10 µL of PCR) <br> ''l1:''50 bp ladder, ''l2-l26:''colonies 7C-9H ]] |
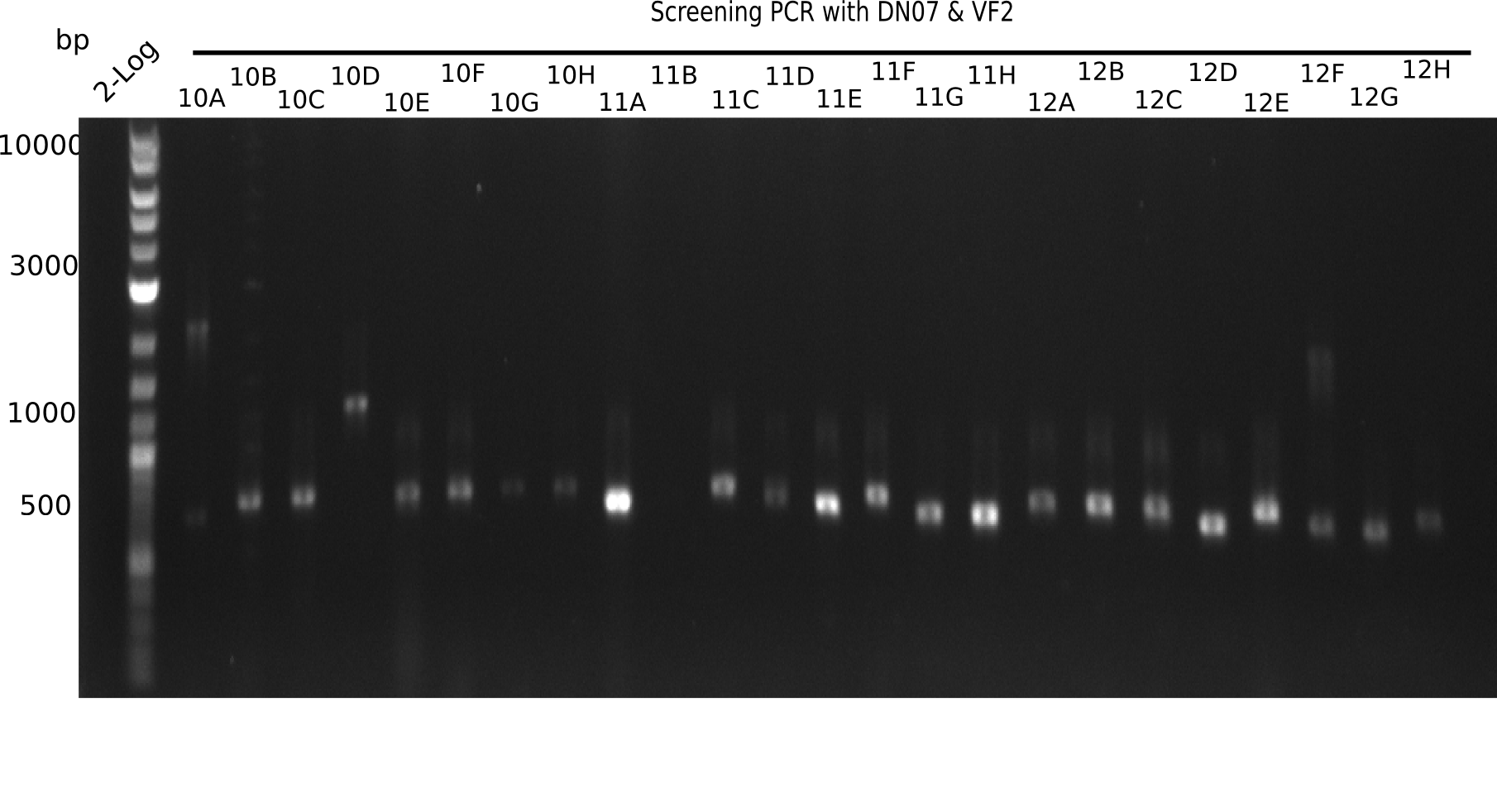
| - | [[File: | + | [[File:Heidelberg_20130916 2log Screen 10-12.png|200px|thumb|right|'''Fig.21.4''' Colony PCR of Gibson assembled DelH-BB without mRFP 96 well plate I (loaded 10 µL of PCR) <br> ''l1:''50 bp ladder, ''l2-l26:''colonies 10A-12H ]] |
<div style="clear:both"></div> | <div style="clear:both"></div> | ||
The following colonies seem positive, because they show the expected band at 663 bp. | The following colonies seem positive, because they show the expected band at 663 bp. | ||
| Line 80: | Line 80: | ||
Expected bands: 11,621, 8,690 and 2,685 bp (PvuI) and 17,801 and 5,105 bp (EcoRI) | Expected bands: 11,621, 8,690 and 2,685 bp (PvuI) and 17,801 and 5,105 bp (EcoRI) | ||
<br/> | <br/> | ||
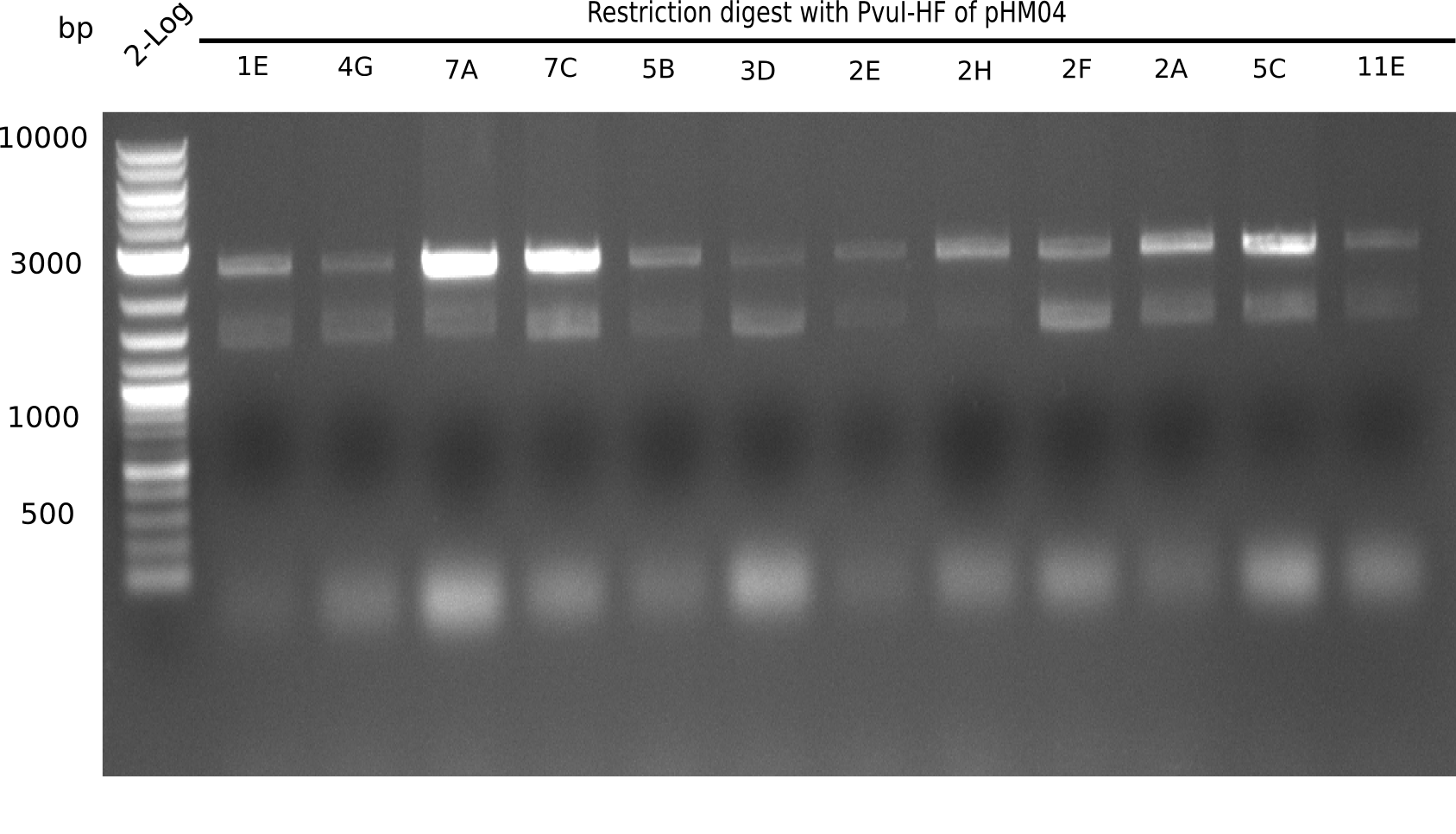
| - | [[File: | + | [[File:Heidelberg_20130917 2log Digest screened colonies.png|200px|thumb|right|'''Fig.21.5''' restriction digest of positive colonies with PvuI (loaded 10 µL of PCR) <br> ''l1:''2 log ladder, ''l2-l13:''colonies 1E-11E ]] |
<div style="clear:both"></div> | <div style="clear:both"></div> | ||
| Line 135: | Line 135: | ||
Expected band: 4. Kb | Expected band: 4. Kb | ||
<br/> | <br/> | ||
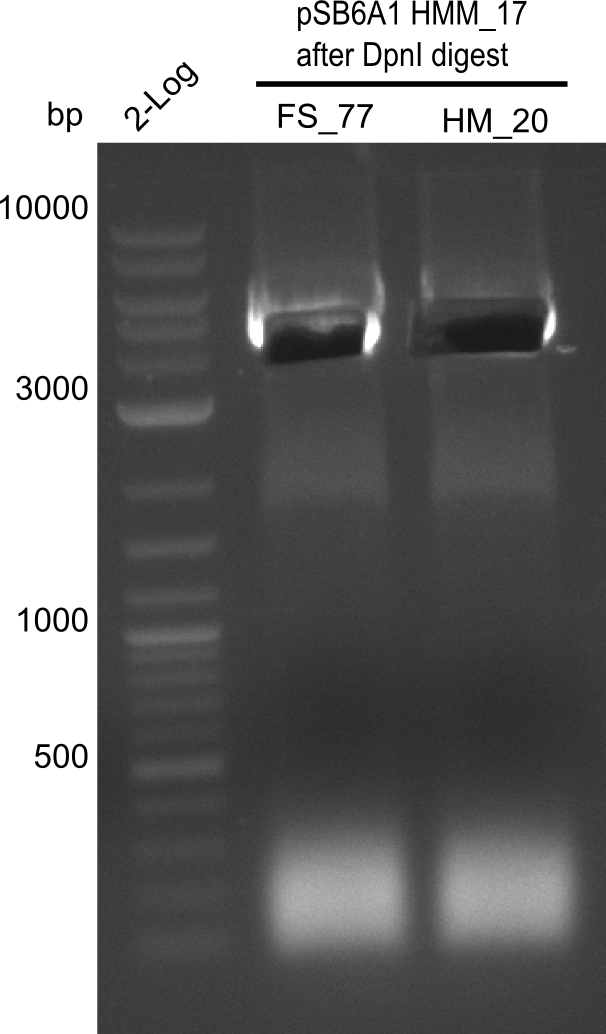
| - | [[File: | + | [[File:Heidelberg_20130918 2log BBHanna FSK1 FSL2 HMK1 HM L2.png|200px|thumb|right|'''Fig.21.6''' Amplification of backbone pSB6A1 directly from two wells (loaded 10 µL of PCR) <br> ''l1:''2log ladder, ''l2''HM20 aus 1K, ''l3''FS77 aus 1K, ''l4''HM20 aus 2L, ''l5''FS77 aus 2L]] |
| - | [[File: | + | [[File:Heidelberg_20130918 2log BBHanna FSK1 FSL2 HMK1 HM L2 cut.png|200px|thumb|right| '''Fig.21.7''' Amplification of backbone pSB6A1 directly from two wells (loaded 10 µL of PCR) <br> ''l1:''2log ladder, ''l2''HM20 aus 1K, ''l3''FS77 aus 1K, ''l4''HM20 aus 2L, ''l5''FS77 aus 2L - was cut out]] |
<div style="clear:both"></div> | <div style="clear:both"></div> | ||
Amplification worked with the template pSB6A1 from the biobrick distribution 2013 plate 2, well L2. | Amplification worked with the template pSB6A1 from the biobrick distribution 2013 plate 2, well L2. | ||
| Line 185: | Line 185: | ||
Expected band: 4.4 Kb | Expected band: 4.4 Kb | ||
<br/> | <br/> | ||
| - | [[File: | + | [[File:Heidelberg_20130918 log2 2x15doubledig 2x6bdoubledig BBFS BBHM.png|200px|thumb|right|'''Fig.21.8''' Amplification of backbone pSB6A1 from colony transformed with pSB6A1 from biobrick distribution 2013 plate 2, well L2 <br> ''l1:''2log ladder, ''l2'' FS_77, ''l3''HM20 after DpnI digest]] |
| - | [[File: | + | [[File:Heidelberg_20130918 log2 2x15doubledig 2x6bdoubledig BBFS BBHM cut.png|200px|thumb|right| '''Fig.21.9''' Amplification of backbone pSB6A1 from colony transformed with pSB6A1 from biobrick distribution 2013 plate 2, well L2 <br> ''l1:''2log ladder, ''l2'' FS_77, ''l3''HM20 after DpnI digest - was cut]] |
<div style="clear:both"></div> | <div style="clear:both"></div> | ||
Gel shows amplification of fragment. | Gel shows amplification of fragment. | ||
| Line 268: | Line 268: | ||
Expected band: 663 bp | Expected band: 663 bp | ||
<br/> | <br/> | ||
| - | [[File: | + | [[File:Heidelberg_20130920 2log 96erColonyNr.1a.png|200px|thumb|right|'''Fig.21.10''' Colony PCR of Gibson assembled DelH-BB without mRFP 96 well plate I(loaded 4 µL of PCR) <br> ''l1:''2-log ladder, ''l2-l26:''colonies A1-C1 ]] |
| - | [[File: | + | [[File:Heidelberg_20130920 2log 96erColonyNr.1b.png|200px|thumb|right|'''Fig.21.11''' Colony PCR of Gibson assembled DelH-BB without mRFP 96 well plate I (loaded 4 µL of PCR) <br> ''l1:''2-log ladder, ''l2-l26:''colonies C2-E2 ]] |
| - | [[File: | + | [[File:Heidelberg_20130920 2log 96erColonyNr.2a.png|200px|thumb|right|'''Fig.21.12''' Colony PCR of Gibson assembled DelH-BB without mRFP 96 well plate I(loaded 4 µL of PCR) <br> ''l1:''2-log ladder, ''l2-l26:''colonies E3-G3 ]] |
| - | [[File: | + | [[File:Heidelberg_20130920 2log 96erColonyNr.2b.png|200px|thumb|right|'''Fig.21.13''' Colony PCR of Gibson assembled DelH-BB without mRFP 96 well plate I (loaded 4 µL of PCR) <br> ''l1:''2-log ladder, ''l2-l26:''colonies G4-H12 ]] |
<div style="clear:both"></div> | <div style="clear:both"></div> | ||
The following colonies seem positive, because they show the expected band at 663 bp | The following colonies seem positive, because they show the expected band at 663 bp | ||
| Line 329: | Line 329: | ||
Expected band: 663 bp | Expected band: 663 bp | ||
<br/> | <br/> | ||
| - | [[File: | + | [[File:Heidelberg_20130920 2log DelH96erColonyNr.5&6a.png |200px|thumb|right|'''Fig.21.14''' Colony PCR of Gibson assembled DelH-BB without mRFP 96 well plate II(loaded 4 µL of PCR) <br> ''l1:''2-log ladder, ''l2-l26:''colonies A1-B12 ]] |
| - | [[File: | + | [[File:Heidelberg_20130920 2log DelH96erColonyNr.5&6b.png|200px|thumb|right|'''Fig.21.15''' Colony PCR of Gibson assembled DelH-BB without mRFP 96 well plate II (loaded 4 µL of PCR) <br> ''l1:''2-log ladder, ''l2-l26:''colonies C1-D12 ]] |
| - | [[File: | + | [[File:Heidelberg_20130920 2log DelH96erColonyNr.7&8a.png|200px|thumb|right|'''Fig.21.16''' Colony PCR of Gibson assembled DelH-BB without mRFP 96 well plate II (loaded 4 µL of PCR) <br> ''l1:''2-log ladder, ''l2-l26:''colonies E1-F12 ]] |
| - | [[File: | + | [[File:Heidelberg_20130920 2log DelH96erColonyNr.7&8b.png|200px|thumb|right|'''Fig.21.17''' Colony PCR of Gibson assembled DelH-BB without mRFP 96 well plate II (loaded 4 µL of PCR) <br> ''l1:''2-log ladder, ''l2-l26:''colonies G1-H12 ]] |
<div style="clear:both"></div> | <div style="clear:both"></div> | ||
The following colonies seem positive, because they show the expected band at 663 bp | The following colonies seem positive, because they show the expected band at 663 bp | ||
| Line 360: | Line 360: | ||
Expected bands: 11,621, 8,628 & 2,685 bp | Expected bands: 11,621, 8,628 & 2,685 bp | ||
<br/> | <br/> | ||
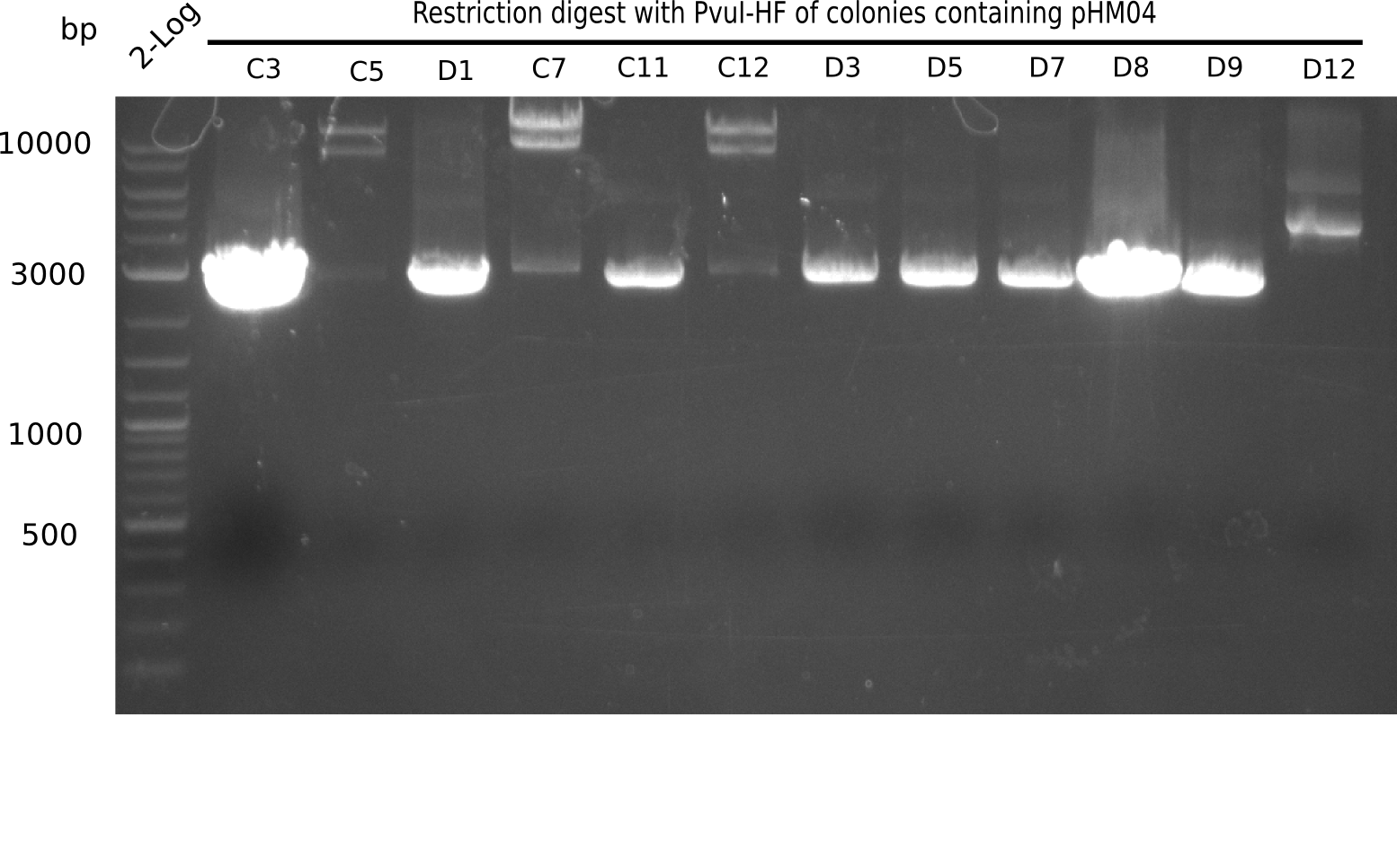
| - | [[File: | + | [[File:Heidelberg_20130922 2log Digest C3-D12.png|200px|thumb|right|'''Fig.21.18''' Test digest with PvuI (loaded 10 µL of PCR) <br> ''l1:'' 2log ladder, ''l2:'' C3,''l3:'' C5, ''l4:'' D1, ''l5:'' C7, ''l6:'' C11,''l7:'' C12, ''l8:'' D3, ''l9:'' D5, ''l10:'' D7,''l11:'' D8, ''l12:''D9, ''l13:''D12 ]] |
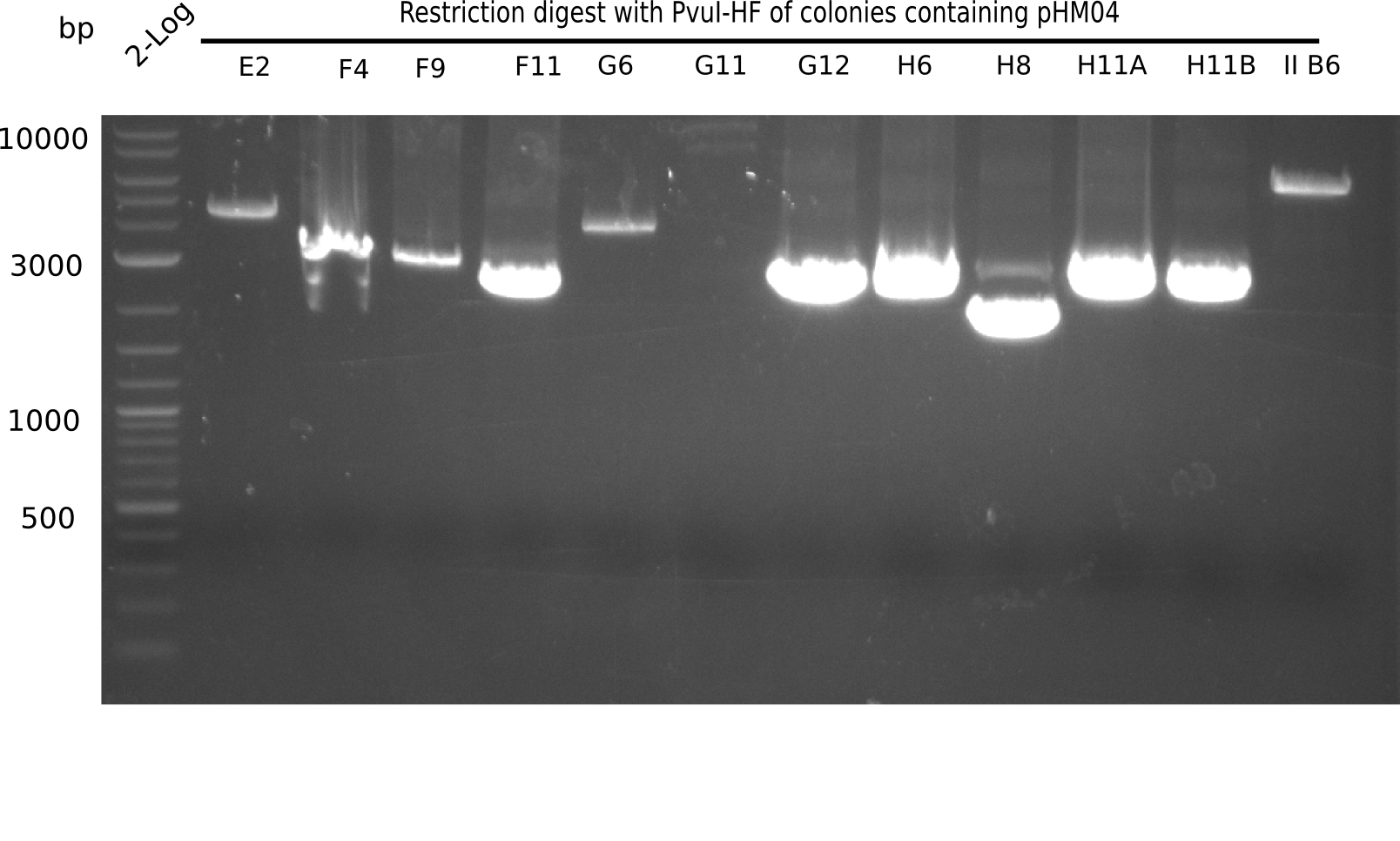
| - | [[File: | + | [[File:Heidelberg_20130922 2log Digest E2-IIB6.png|200px|thumb|right|'''Fig.21.19''' Test digest with PvuI (loaded 10 µL of PCR) <br> ''l1:'' 2log ladder, ''l2:'' E2,''l3:'' F4, ''l4:'' F9, ''l5:'' F11, ''l6:'' G6,''l7:'' G11, ''l8:'' G12 ''l9:'' H6, ''l10:'' H8,''l11:'' H11A, ''l12:''H11B, ''l13:''II B6 ]] |
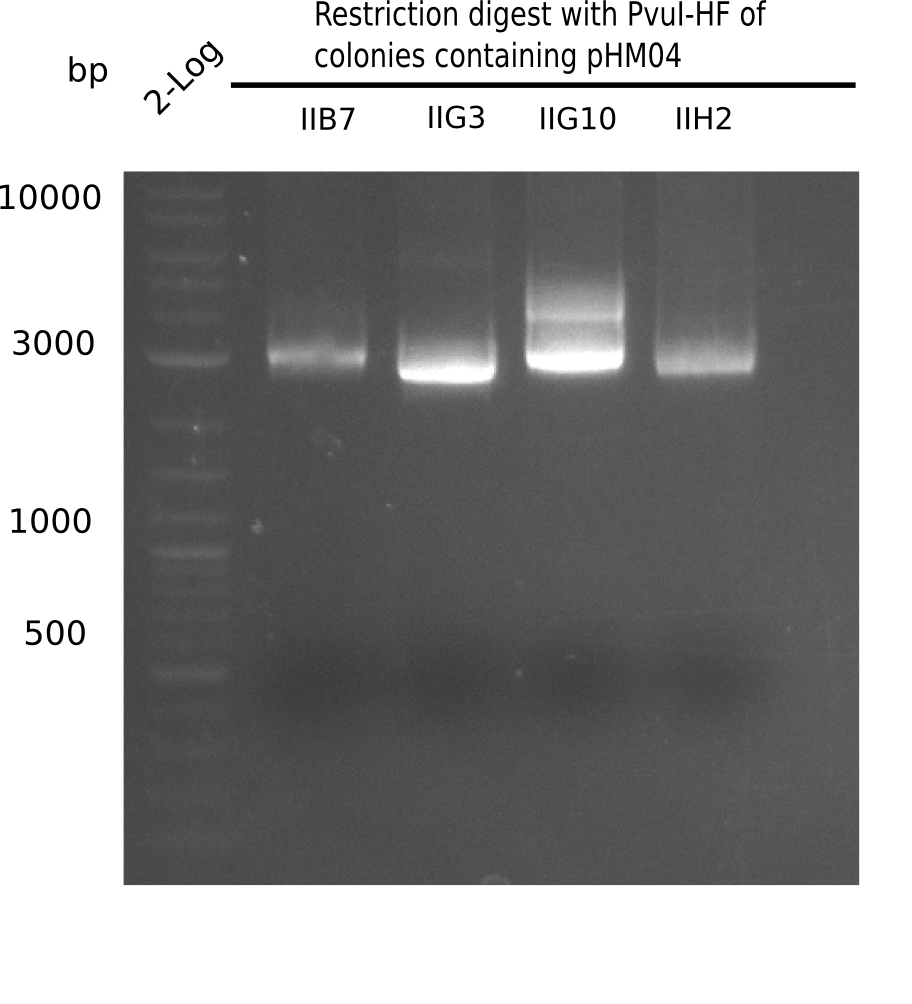
| - | [[File: | + | [[File:Heidelberg_20130922 2log Digest IIB7-IIH2.png|200px|thumb|right|'''Fig.21.20''' Test digest with PvuI (loaded 10 µL of PCR) <br> ''l1:'' 2log ladder, ''l2:'' II B7,''l3:'' II G3, ''l4:'' II G10, ''l5:''II H2]] |
<div style="clear:both"></div> | <div style="clear:both"></div> | ||
The following colonies show the correct pattern: | The following colonies show the correct pattern: | ||
| Line 390: | Line 390: | ||
! Colonies !! Alignment File | ! Colonies !! Alignment File | ||
|- | |- | ||
| - | | I C5 || [[File: | + | | I C5 || [[File:Heidelberg_Sequencing Result pHM04 DN07 colony 05 old.clustal | Sequencing of clone I C5 with DN_07]] |
|- | |- | ||
| I C7 || sequencing insufficient | | I C7 || sequencing insufficient | ||
| Line 449: | Line 449: | ||
<br/> | <br/> | ||
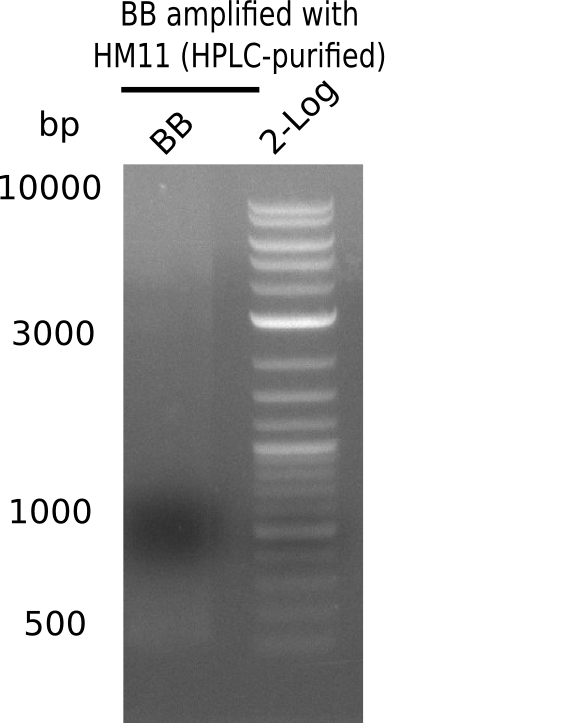
| - | [[File: | + | [[File:Heidelberg_20130920 HM11-BB 2log.png|200px|thumb|right|'''Fig.21.21''' gel of amplified BB with HM11 (loaded 20 µL of PCR) <br> ''l1:'' BB amplified with HM11 HPLC-purified at 68°C td ''l2:'' 2log<br> no product]] |
<div style="clear:both"></div> | <div style="clear:both"></div> | ||
Gel does not show amplified backbone fragment. | Gel does not show amplified backbone fragment. | ||
| Line 491: | Line 491: | ||
Expected band: ~ 4.4 Kb | Expected band: ~ 4.4 Kb | ||
<br/> | <br/> | ||
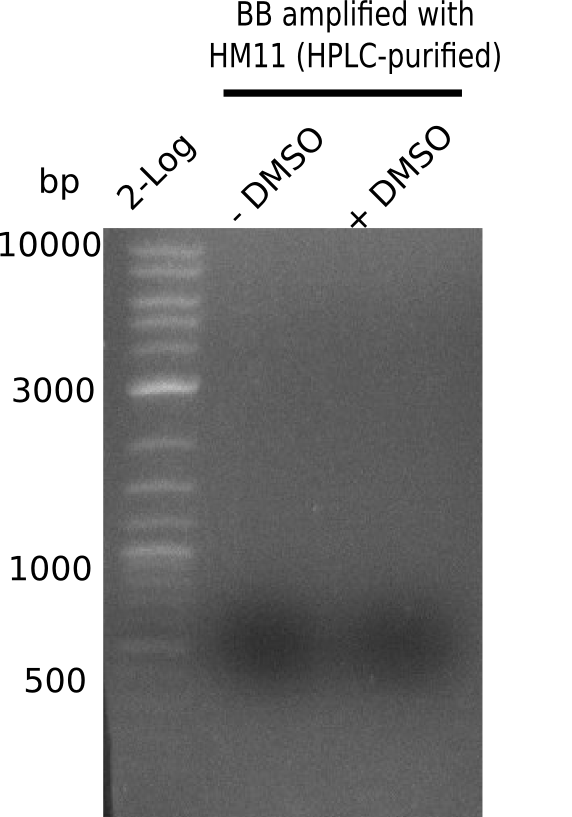
| - | [[File: | + | [[File:Heidelberg_20130921 2log HM11-BB DMSO.png|200px|thumb|right|'''Fig.21.22''' Gel of BB amplified with HM11: ''l1:''2 log ladder, ''l2:'' BB without DMSO, ''l3:'' BB with DMSO ]] |
<div style="clear:both"></div> | <div style="clear:both"></div> | ||
Gel shows strong and specific amplification of backbone. | Gel shows strong and specific amplification of backbone. | ||
| Line 535: | Line 535: | ||
===New HPLC Purified Primers=== | ===New HPLC Purified Primers=== | ||
As pointed out before, we suspect DelH to be toxic for ''E. coli'' and therefore, only colinies containing mutated constructs survive. To avoid this, we ordered new shorter and HPLC purified primers for the assembly of pHM04. | As pointed out before, we suspect DelH to be toxic for ''E. coli'' and therefore, only colinies containing mutated constructs survive. To avoid this, we ordered new shorter and HPLC purified primers for the assembly of pHM04. | ||
| - | [[File: | + | [[File:Heidelberg_pHM_04.png|300px|left|thumb|200px|Vector map of the [[:File:Heidelberg_PHM04-DelH-pSB6A1(without mRFP).gb|PHM04-DelH-pSB6A1(without mRFP) Gibson plasmid]].]] |
{| class="wikitable" style="float" | {| class="wikitable" style="float" | ||
|- | |- | ||
| Line 570: | Line 570: | ||
====Purification of Midiprep of Clone I6B==== | ====Purification of Midiprep of Clone I6B==== | ||
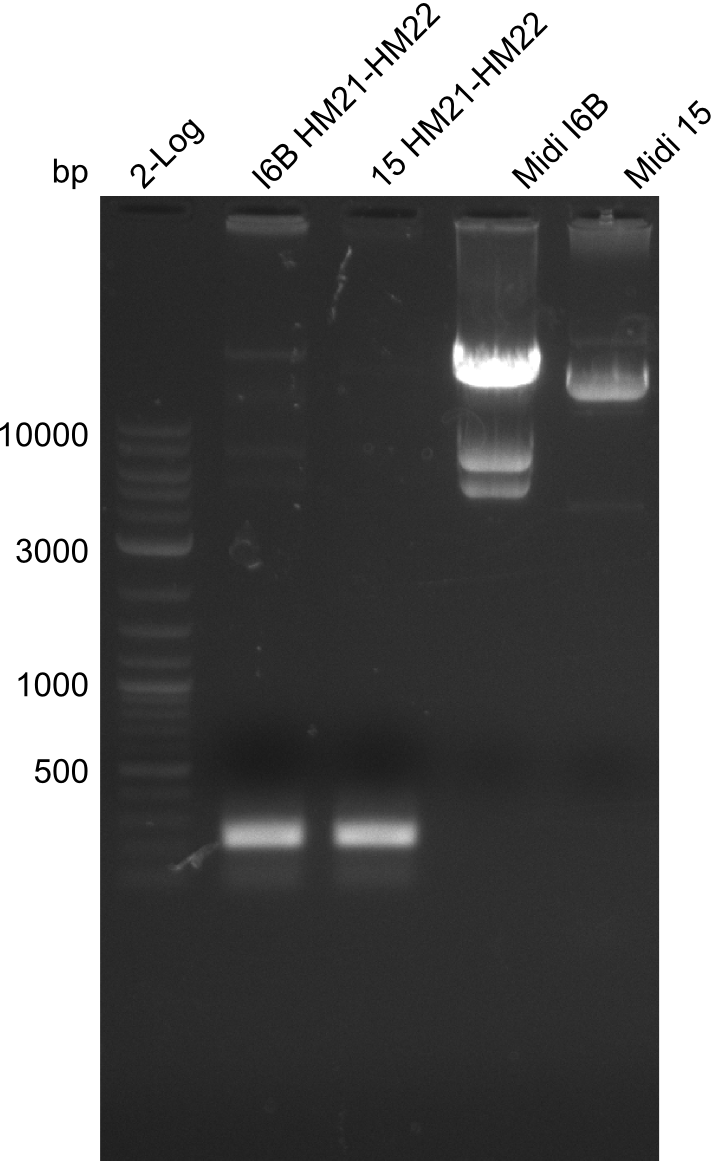
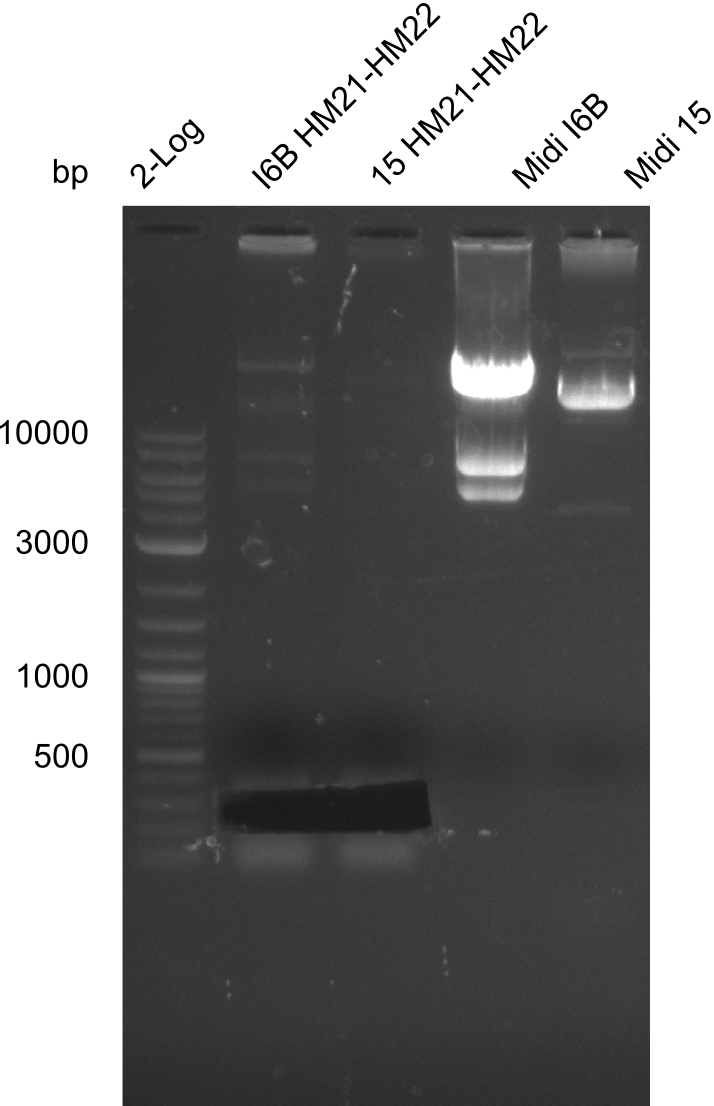
| - | [[File: | + | [[File:Heidelberg_20130918 2log 6BHM21-HM22 15HM21-HM22 midi6B maxi15 log2 pIK8.6 pSB1C3 log HM20 FS20.png|200px|thumb|'''Fig.21.23''' Midipreps of colonies I6B and 15]] |
As can be seen in figure 21.23, the midi-prep of colony I 6B is contaminated. Thus, we tried to purify only the largest band by gel extraction with the QIAEX II Gel Extraction Kit (Qiagen). The procedure was as following: | As can be seen in figure 21.23, the midi-prep of colony I 6B is contaminated. Thus, we tried to purify only the largest band by gel extraction with the QIAEX II Gel Extraction Kit (Qiagen). The procedure was as following: | ||
* Application of 2 µl of midiprep on gel (4596.7 ng/µl --> ~10 µg) | * Application of 2 µl of midiprep on gel (4596.7 ng/µl --> ~10 µg) | ||
| Line 676: | Line 676: | ||
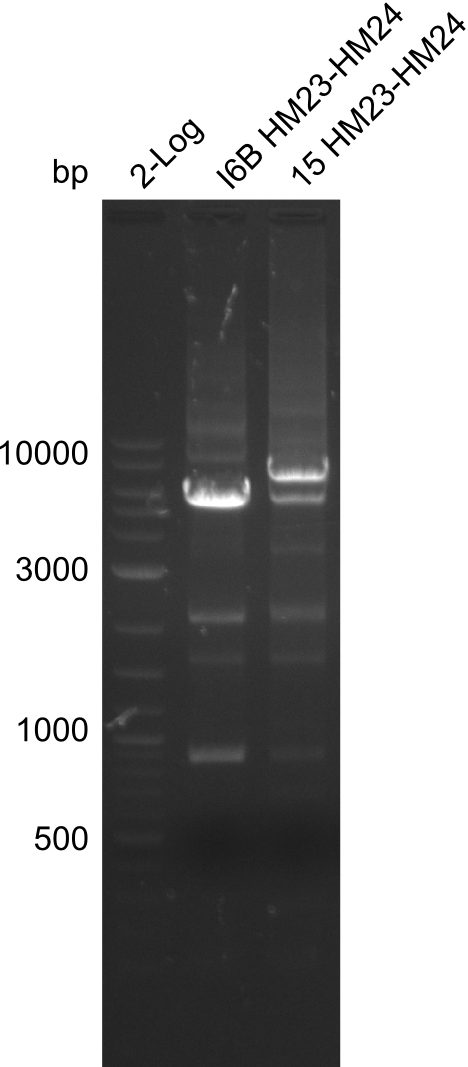
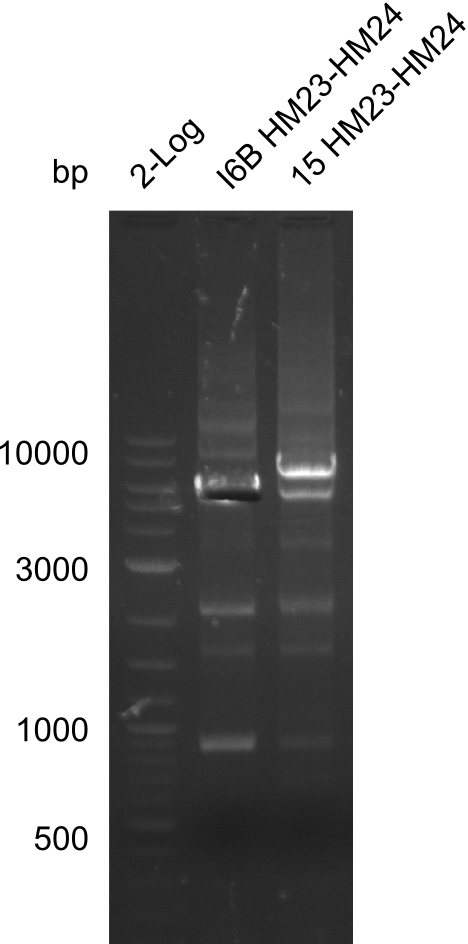
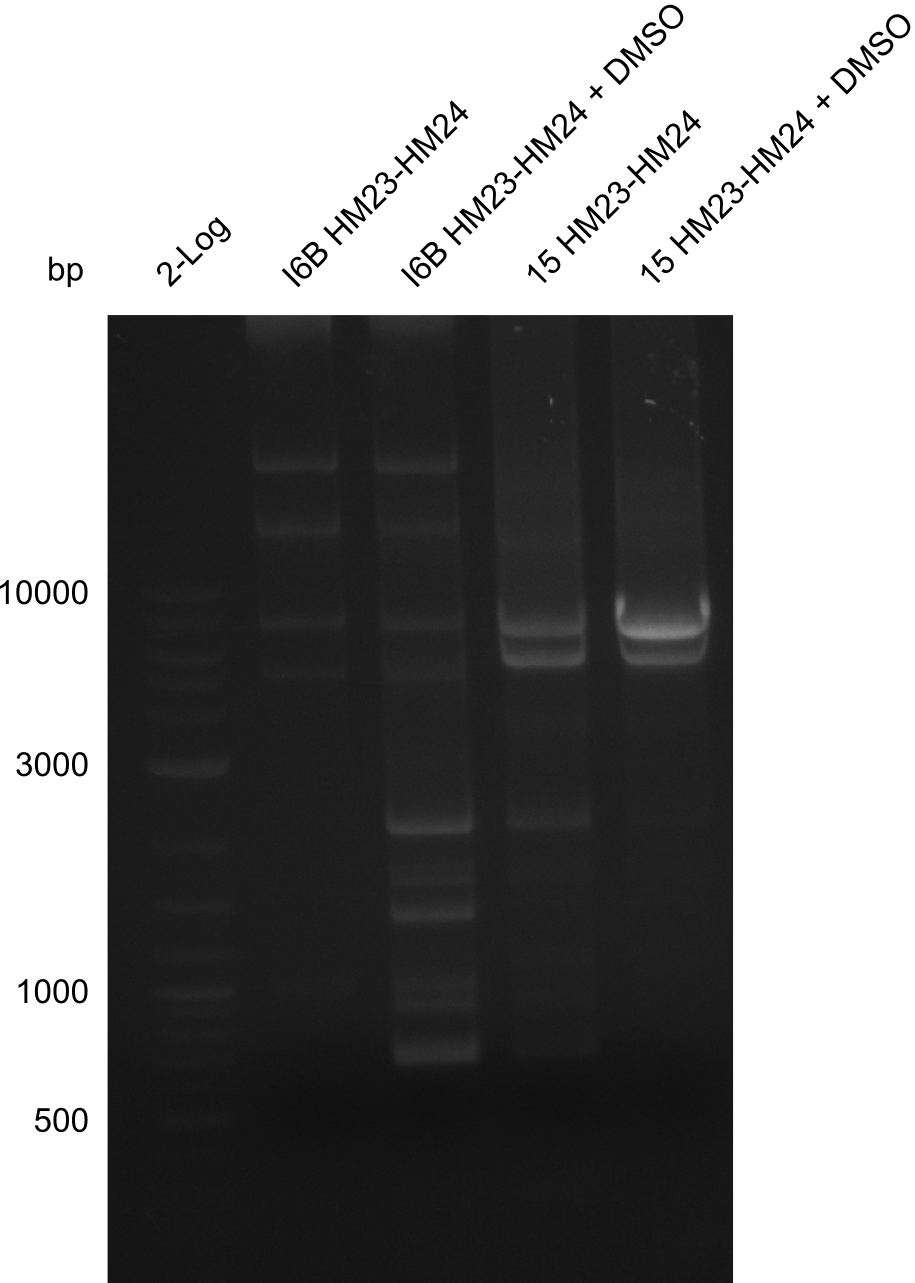
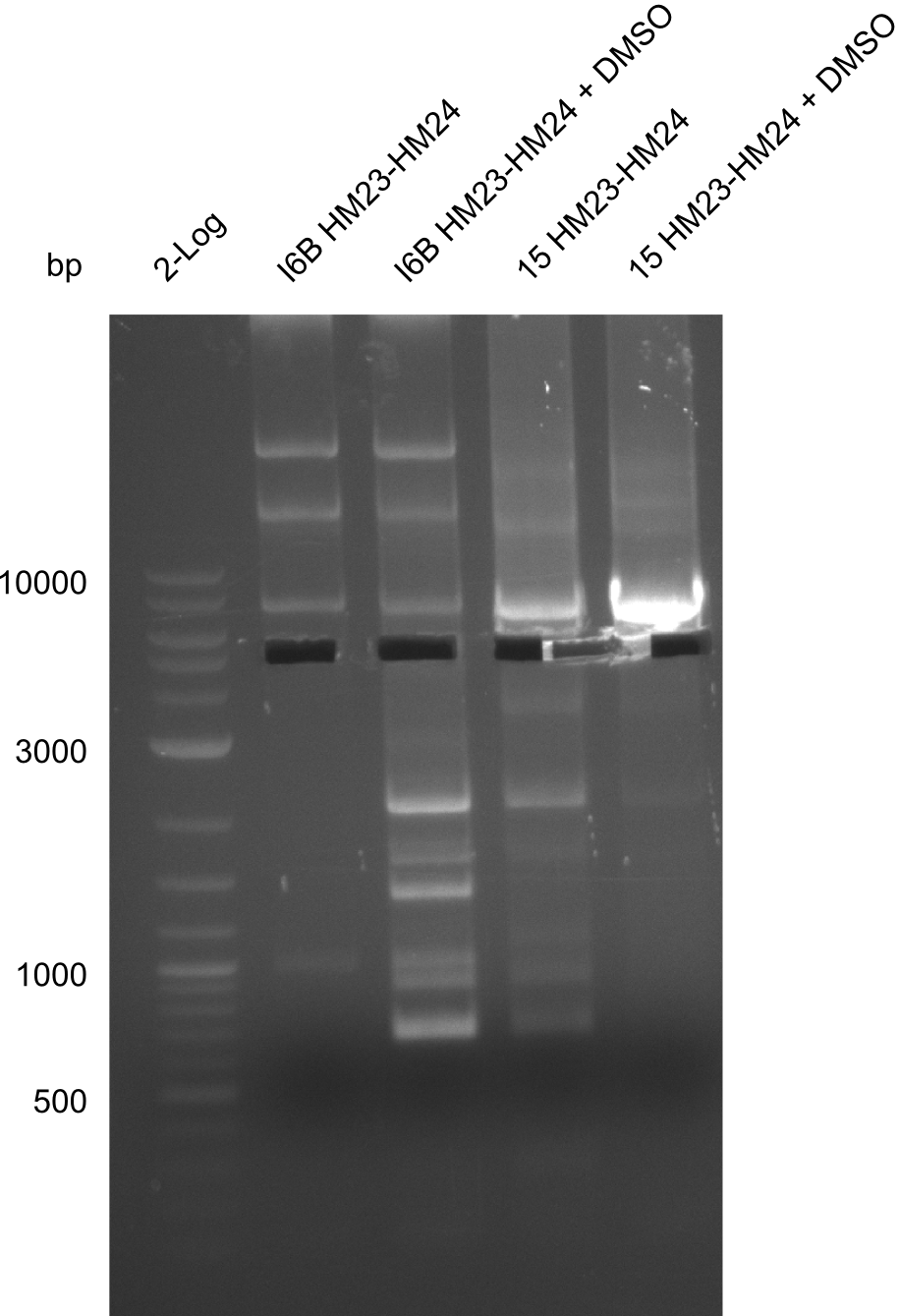
Gels for amplification of HM23 to HM24 | Gels for amplification of HM23 to HM24 | ||
<gallery> | <gallery> | ||
| - | File: | + | File:Heidelberg_20130917 2log 16Bmut 15mut pSB6A1HM pSB6A1FS.png | '''Fig.21.24''' |
| - | File: | + | File:Heidelberg_20130917 2log 16Bmut 15mut pSB6A1HM pSB6A1FS cut.png | '''Fig.21.25''' |
| - | File: | + | File:Heidelberg_20130917 1kb pSB1C3 2log 6BVerd 15Verd 6BPCR 15PCR 1kb.png| '''Fig.21.26''' |
| - | File: | + | File:Heidelberg_20130917 1KB PSB1C3 2LOG 6BVERD 15VERD 6BPCR 15PCR 1KB cut.png | '''Fig.21.27''' |
| - | File: | + | File:Heidelberg_20130918 log2 I6b(23-24) I6b(23-24)DMSO 15(23-24) 15(23-24)DMSO.png | '''Fig.21.28''' |
| - | File: | + | File:Heidelberg_20130918 log2 I6b(23-24) I6b(23-24)DMSO 15(23-24) 15(23-24)DMSOcut.png | '''Fig.21.29''' |
</gallery> | </gallery> | ||
<div style="clear:both"></div> | <div style="clear:both"></div> | ||
| Line 722: | Line 722: | ||
Gels for amplification of HM21 to HM22 | Gels for amplification of HM21 to HM22 | ||
<gallery> | <gallery> | ||
| - | File: | + | File:Heidelberg_20130918 2log 6BHM21-HM22 15HM21-HM22 midi6B maxi15 log2 pIK8.6 pSB1C3 log HM20 FS20.png | '''Fig.21.30''' |
| - | File: | + | File:Heidelberg_20130918 2log 6BHM21-HM22 15HM21-HM22 midi6B maxi15 log2 pIK8.6 pSB1C3 log HM20 FS20cut.png | '''Fig.21.31''' |
</gallery> | </gallery> | ||
<div style="clear:both"></div> | <div style="clear:both"></div> | ||
| Line 749: | Line 749: | ||
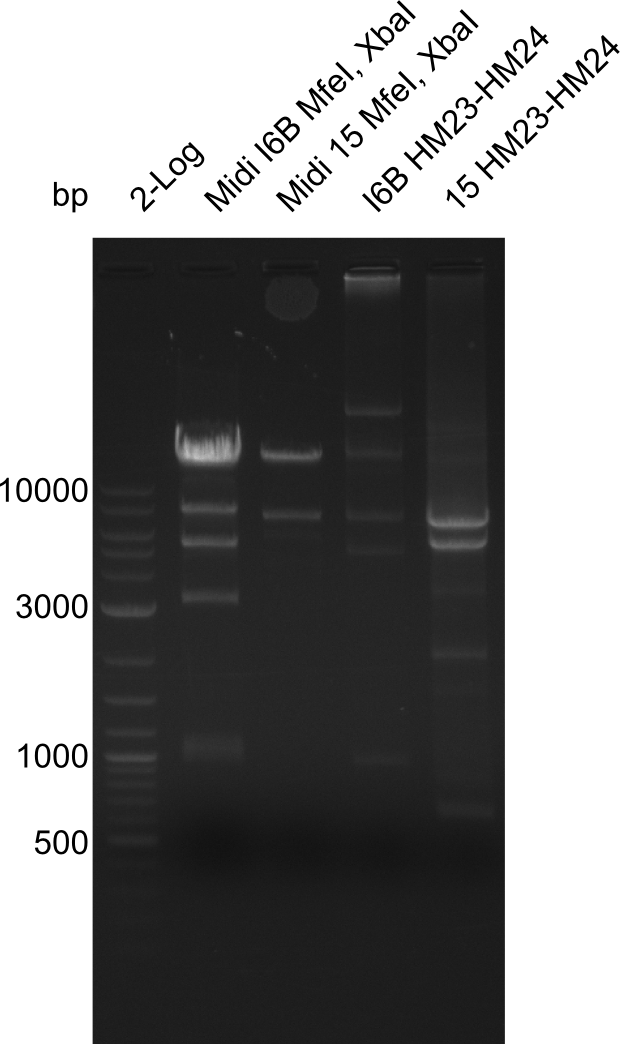
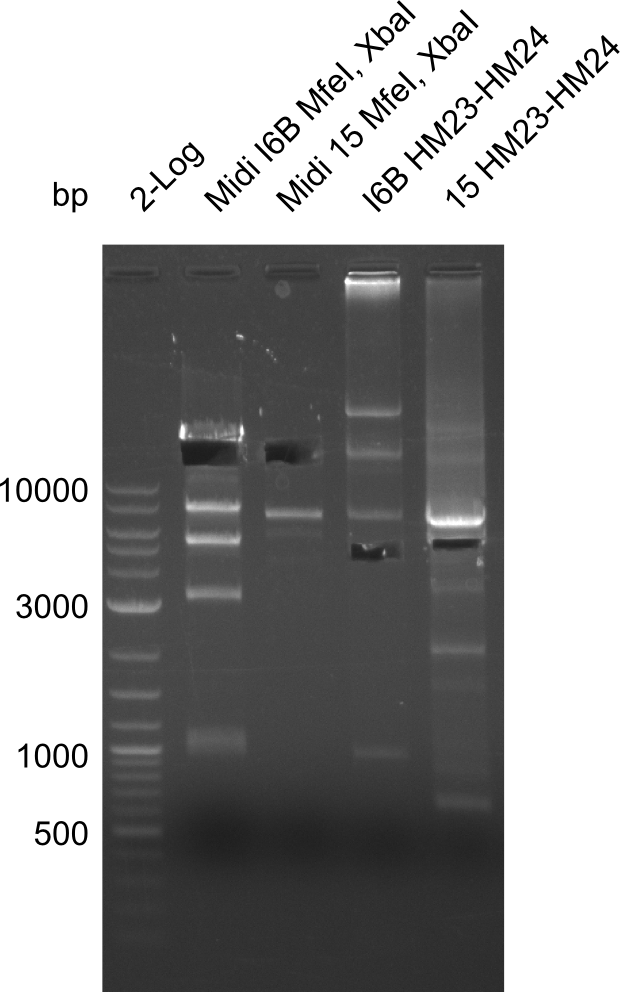
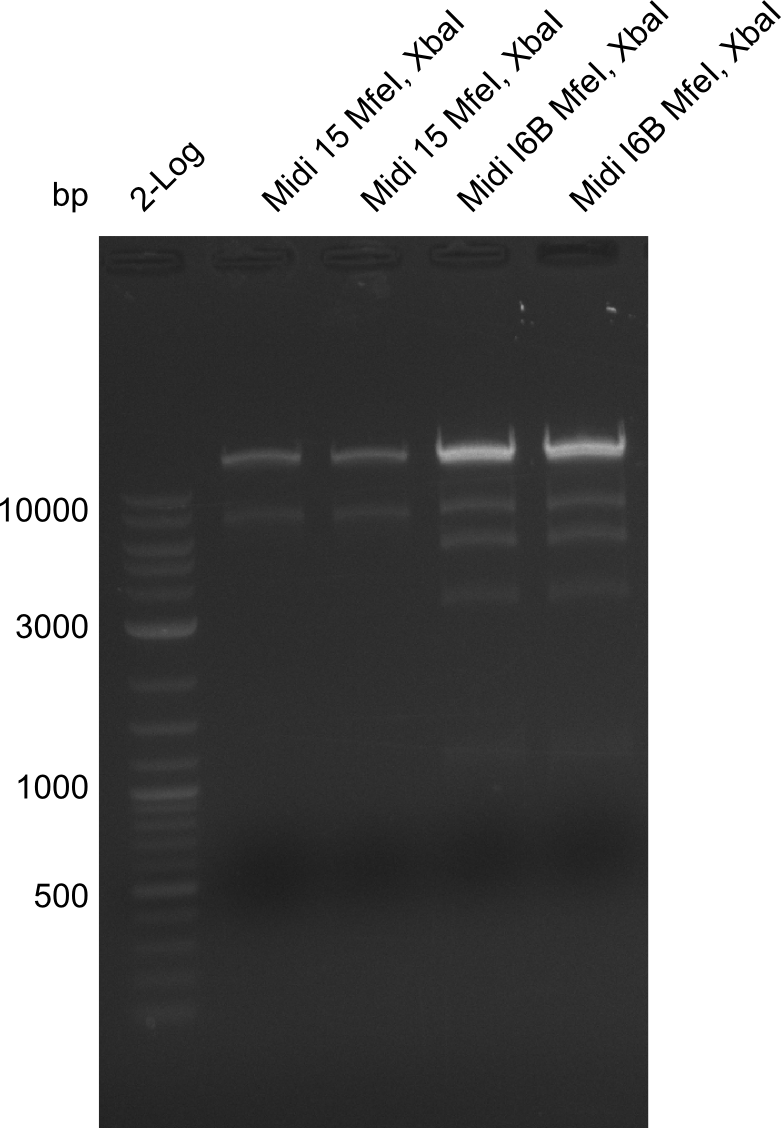
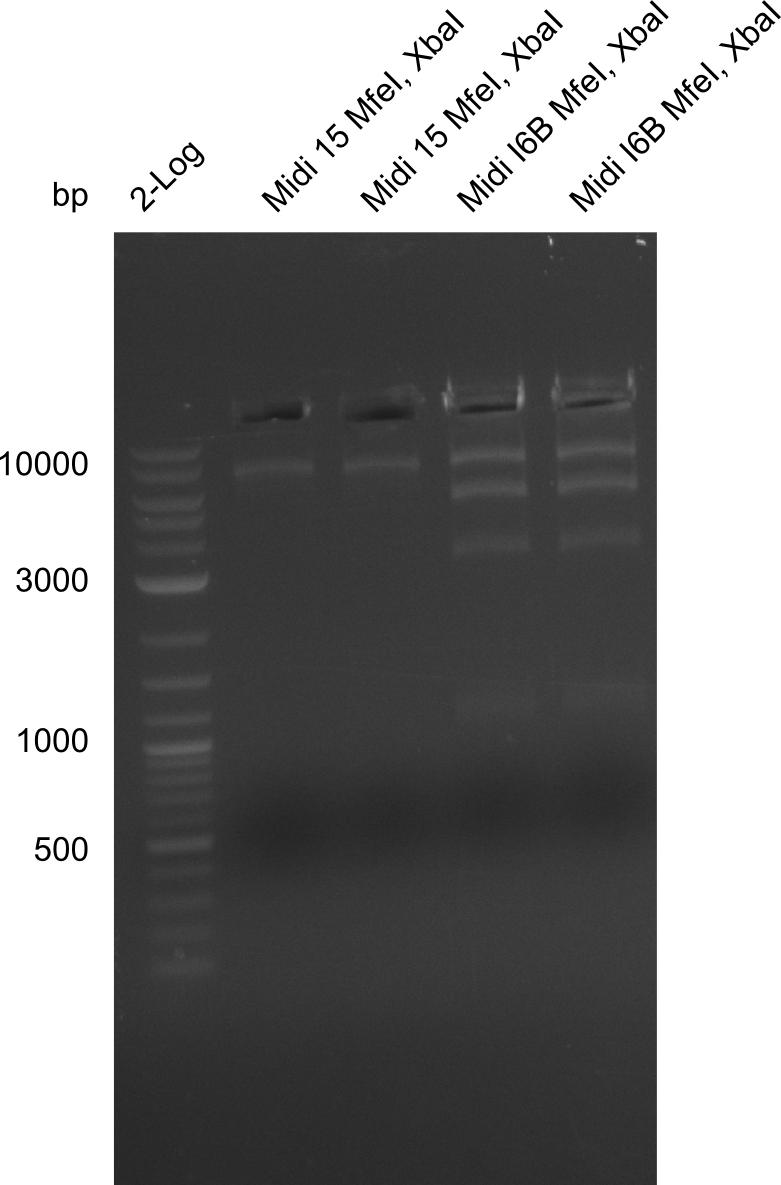
Gels for restriction Digest of pHM04 (I6B) with XbaI and MfeI | Gels for restriction Digest of pHM04 (I6B) with XbaI and MfeI | ||
<gallery> | <gallery> | ||
| - | File: | + | File:Heidelberg_20130917 1kb pSB1C3 2log 6BVerd 15Verd 6BPCR 15PCR 1kb.png| '''Fig.21.32''' |
| - | File: | + | File:Heidelberg_20130917 1KB PSB1C3 2LOG 6BVERD 15VERD 6BPCR 15PCR 1KB cut.png | '''Fig.21.33''' |
| - | File: | + | File:Heidelberg_20130918 log2 2x15doubledig 2x6bdoubledig.png | '''Fig.21.34''' |
| - | File: | + | File:Heidelberg_20130918 log2 2x15doubledig 2x6bdoubledigcut.png | '''Fig.21.35''' |
</gallery> | </gallery> | ||
<div style="clear:both"></div> | <div style="clear:both"></div> | ||
| Line 799: | Line 799: | ||
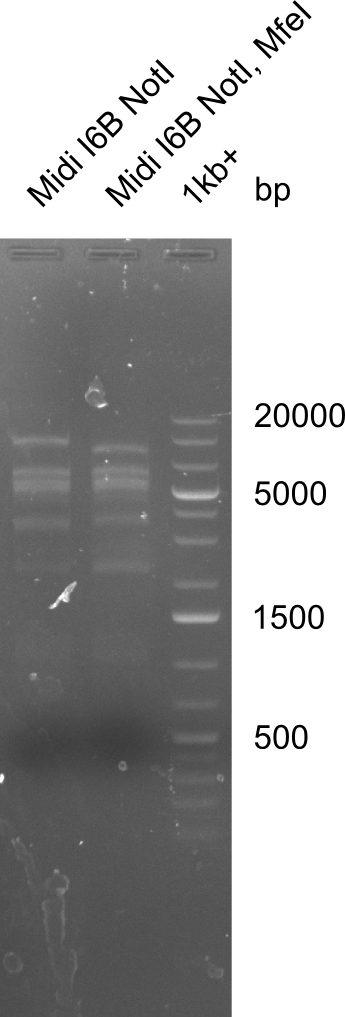
====Test for MfeI Cutting Site==== | ====Test for MfeI Cutting Site==== | ||
| - | [[File: | + | [[File:Heidelberg_20130919 1kb I6BNotI I6BNotIMfeI.png|100px|thumb|'''Fig.21.36''' Test if MfeI restriction site is present]] |
{| class="wikitable" style="float:left; margin-right:1em" | {| class="wikitable" style="float:left; margin-right:1em" | ||
|- | |- | ||
Revision as of 21:11, 1 October 2013
16-09 - 22-09-13
Generation of DelH Plasmid pHM04 15-09
Colony-PCR Conditions CP.W21.A
| Template | 96x 1 picked colony |
| Expected length [bp] | 663 |
| Named | 1A - 12H |
| Primer fw 10 µM | 100x 2 µl VF2 |
| Primer rev 10 µM | 100x 2 µl DN07 |
| iTaq Polymerase (2x) | 100x 10 µl |
| ddH2O | 96x 6 µl |
| Cycles | Temperature DelH [°C] | Time [s] |
|---|---|---|
| 1 | 95 | 120 |
| 12 | 95 | 30 |
| 68 (touchdown -0.5°C) | 30 | |
| 72 | 45 | |
| 18 | 95 | 30 |
| 65 | 30 | |
| 72 | 45 | |
| 1 | 12 | inf |
Result
Expected band: 663 bp
The following colonies seem positive, because they show the expected band at 663 bp.
| Picture | Positive colonies |
|---|---|
| 21.1 | 1B, 1C, 1E, 1F, 1H, 2A, 2B, 2D, 2E, 2F, 3A, 3B, 3C, 3D, 3F, 3G |
| 21.2 | 4A, 4C, 4D, 4G, 5A, 5B, 5C, 5D, 5E, 6A, 6B, 6C, 6E, 6F |
| 22.3 | 7A, 7B, 7C, 7D, 8A, 8B, 8C, 9A, 9E, 9F |
| 22.4 | 11 A, 11E, 11G, 11H, 12D, 12E |
Test Restriction Digest
24 of these positive screened colonies were minipreped and digested with PvuI
| Sample | CutSmartBuffer | PvuI |
|---|---|---|
| 8.5 µl of colonies | 1 µl | 0.5 µl |
Another restriction digest was performed. Colony 7D was digested with EcoRI-HF
| Sample | CutSmartBuffer | EcoRI-HF |
|---|---|---|
| 8.5 µl of colony 7D | 1 µl | 0.5 µl |
Result
Expected bands: 11,621, 8,690 and 2,685 bp (PvuI) and 17,801 and 5,105 bp (EcoRI)
None of the analyzed clones shows correct band pattern.
- => Clones are discarded.
Amplification of Backbone
New Template
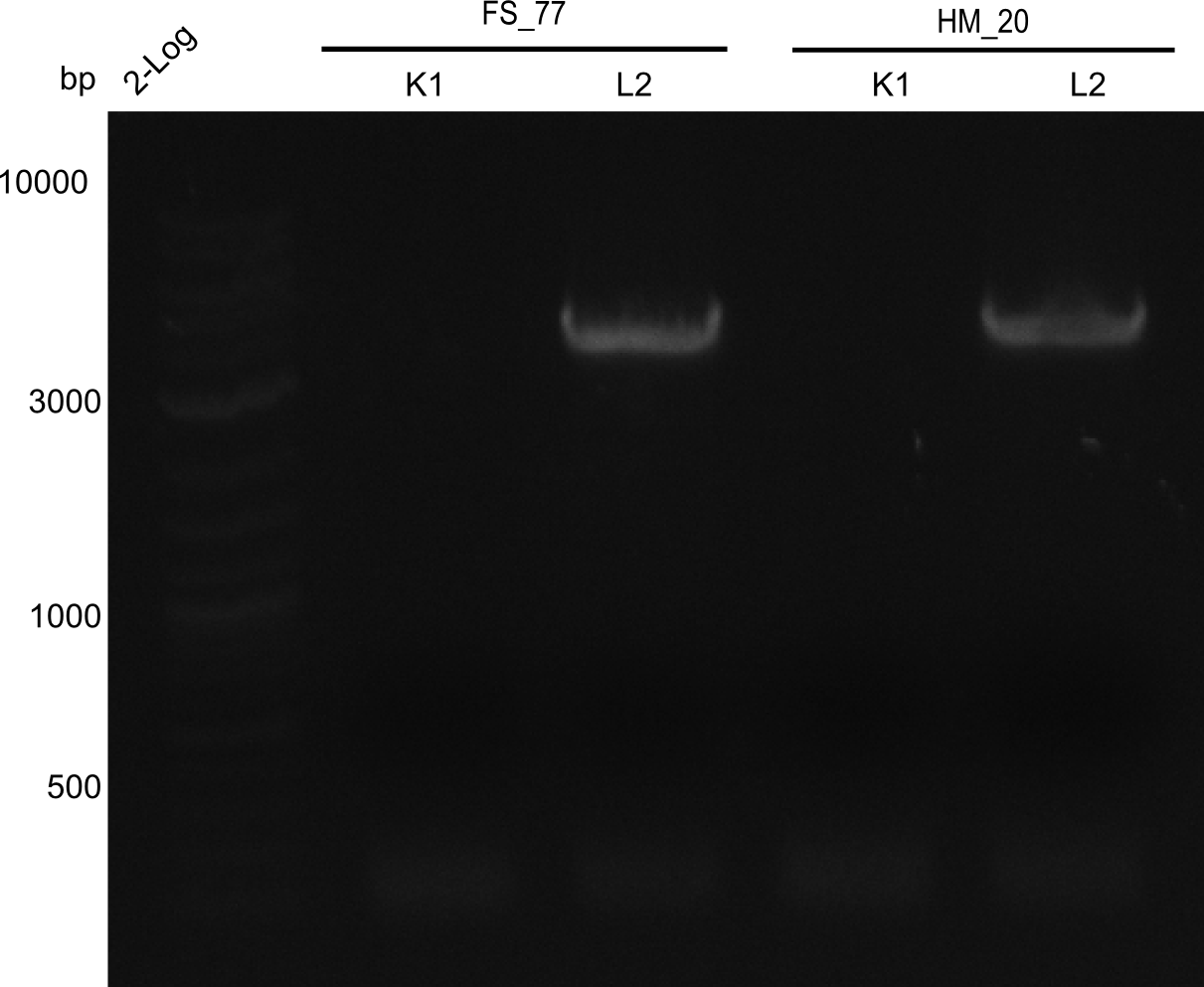
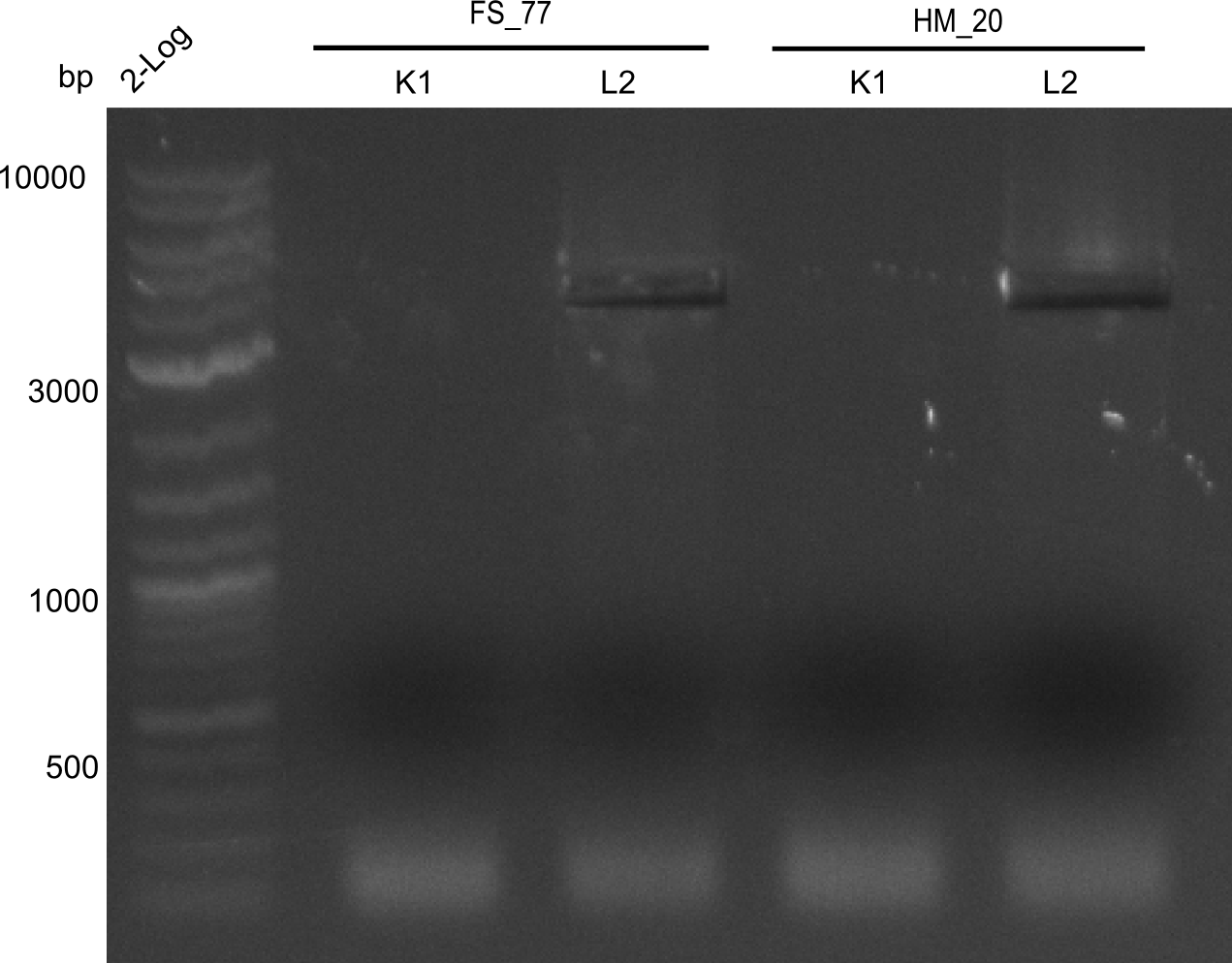
Because the backbone amplification yield from pSB6A1 + BBa_J04450 from Spring distribution 2012 (plate 1, well K1) was so low and the screening did not result in positive restriction digests with PvuI or EcoRI, we go a step back and transform E.coli ToP10 with a new pSB6A1 part from the 2013 distribution. Therefore, we transform on the one hand with the part on plate 2 well 2L and on the other hand with the part from the plate 5 well 1K from the Spring 1023 diestricbution.
Aditionally we ran a PCR directly from the two wells with the backbone primers (HM20, HM17 & FS77).
PCR Conditions BB.W21.A
Amplification of pSB6A1 directly from the parts registry
1x PCR with HM_20 and 1x PCR with FS_77
| Reagent | BB pSB6A1 | BB pSB6A1 | BB pSB6A1 | BB pSB6A1 |
|---|---|---|---|---|
| Expected length [Kb] | 4.4 | 4.4 | 4.4 | 4.4 |
| Template | 1 µl pSB6A1 plate 5, well K1 | 1 µl pSB6A plate 2, well L2 | 1 µl pSB6A1 plate 5, well K1 | 1 µl pSB6A plate 2, well L2 |
| Primer 10 µM fw | 1 µl HM_17 | 1 µl HM_17 | 1 µl HM_17 | 1 µl HM_17 |
| Primer 10 µM rev | 1 µl FS_77 | 1 µl FS_77 | 1 µl HM_20 | 1 µl HM_20 |
| Phusion Flash Master Mix (2x) | 10 | 10 | 10 | 10 |
| DMSO | 1 | 1 | 1 | 1 |
| ddH2O | 6 | 6 | 6 | 6 |
| Cycles | Temperature [°C] | Time [s] |
|---|---|---|
| 1 | 98 | 10 |
| 30 | 98 | 1 |
| 58 | 5 | |
| 72 | 80 | |
| 1 | 72 | 5:00 min |
| 1 | 4 | inf |
Result
Expected band: 4. Kb
Amplification worked with the template pSB6A1 from the biobrick distribution 2013 plate 2, well L2.
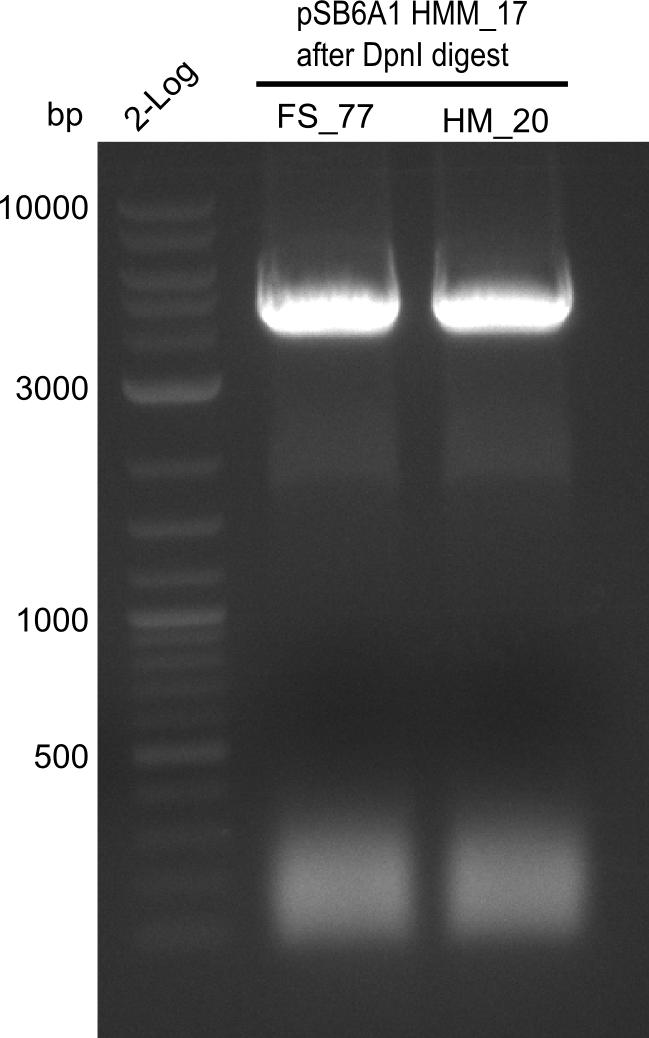
- => The fragment was gel extracted with QIAquick gel extraction kit, digested with DpnI, and gel extracted again, resulting in final concentrations of 10.7 ng/µl for FS_77 and 4.8 ng/µL for HM_20
- => PCR will be repeated with several samples from the colonies transformed with pSB6A1 from the biobrick distribution 2013 plate 2, well L2
PCR Conditions BB.W21.A
Amplification of backbone fragment pSB6A1 from colonies of DH10ß, transformed with pSB6A1 from the biobrick distribution 2013 plate 2, well L2
4x PCR with HM_20 and 4x PCR with FS_77
| Reagent | BB pSB6A1 | BB pSB6A1 |
|---|---|---|
| Expected length [Kb] | 4.4 | 4.4 |
| Template | colony of DH10B transformed with pSB6A1 plate 5, well K1 | colony of DH10B transformed with pSB6A1 plate 5, well K1 |
| Primer 10 µM fw | 1 µl HM_17 | 1 µl HM_17 |
| Primer 10 µM rev | 1 µl FS_77 | 1 µl FS_77 |
| Phusion Master Mix (2x) | 10 | 10 |
| DMSO | 1 | 1 |
| ddH2O | 6 | 6 |
| Cycles | Temperature [°C] | Time [s] |
|---|---|---|
| 1 | 98 | 10 |
| 30 | 98 | 1 |
| 58 | 5 | |
| 72 | 80 | |
| 1 | 72 | 5:00 min |
| 1 | 4 | inf |
Result
Expected band: 4.4 Kb
Gel shows amplification of fragment.
- => Fragment was cut and gel extracted, then DpnI digested and gel purified again.
Generation of DelH Plasmid pHM04 18-09
Gibson Assembly
| Fragment | Concentration [ng/µl] | Amount with BB16 (FS77) [µl] | Amount with BB16 (HM20) [µl] |
|---|---|---|---|
| DN11-FS66 | 172.5 | 1.77 | 1.84 |
| FS67-FS68 | 120.7 | 2.15 | 2.24 |
| FS69-FS70 | 211.3 | 2.12 | 2.21 |
| FS71-HM08 | 146 | 1.24 | 1.30 |
| BB16 amplified with FS77 | 43.1 | 2.72 | - |
| BB16 amplified with HM20 | 35.8 | - | 2.42 |
- Incubate for 1 h at 50°C
Electroporation
- Afterwards 3 µl of each Gibson assembly were taken and 2 µl ddH2O were added
- With 17 µl of the Gibson assembly, isopropanol purification was performed, DNA was eluted in 20 µl ddH2O
- 6 different electroporations in E.coli BL21 DE3 were performed and grown at RT (see scheme below)
| Electroporation name | Inserted amount of Gibson Assembly | Plated out on agar plates |
|---|---|---|
| HM20 1 | 3 µl (Gibson + ddH2O) | * 10 µl * rest |
| HM20 20 | 20 µl (isopropanol purified) | * 10 µl * rest |
| FS77 1 | 1 µl (Gibson + ddH2O) | * 10 µl * rest |
| FS77 20 | 20 µl (isopropanol purified) | * 10 µl * rest |
Colony-PCR CP.W21.A
| Template | 96x 1 picked colony |
| Expected length [bp] | 663 |
| Named | 1A - 12H DelH I |
| Primer fw 10 µM | 100x 2 µl VF2 |
| Primer rev 10 µM | 100x 2 µl DN07 |
| One-Taq Polymerase (2x) | 100x 10 µl |
| ddH2O | 96x 6 µl |
| Cycles | Temperature [°C] | Time [s] |
|---|---|---|
| 1 | 95 | 120 |
| 12 | 95 | 30 |
| 68 (touchdown -0.5°C) | 30 | |
| 72 | 45 | |
| 18 | 95 | 30 |
| 65 | 30 | |
| 72 | 45 | |
| 1 | 12 | inf |
Result
Expected band: 663 bp
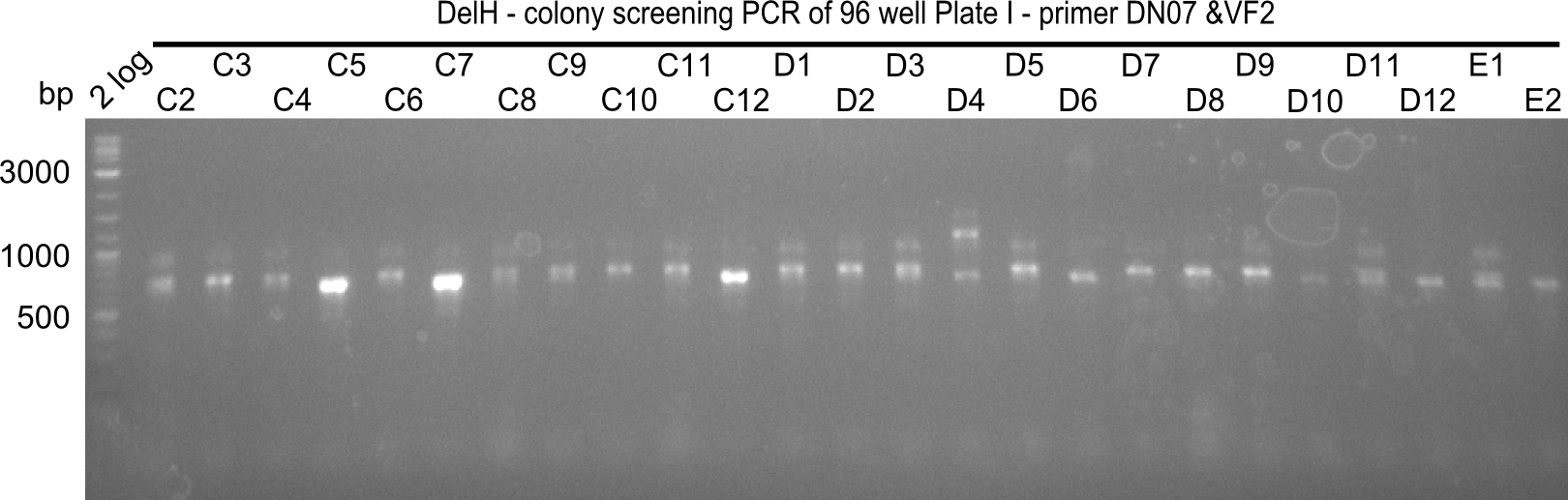
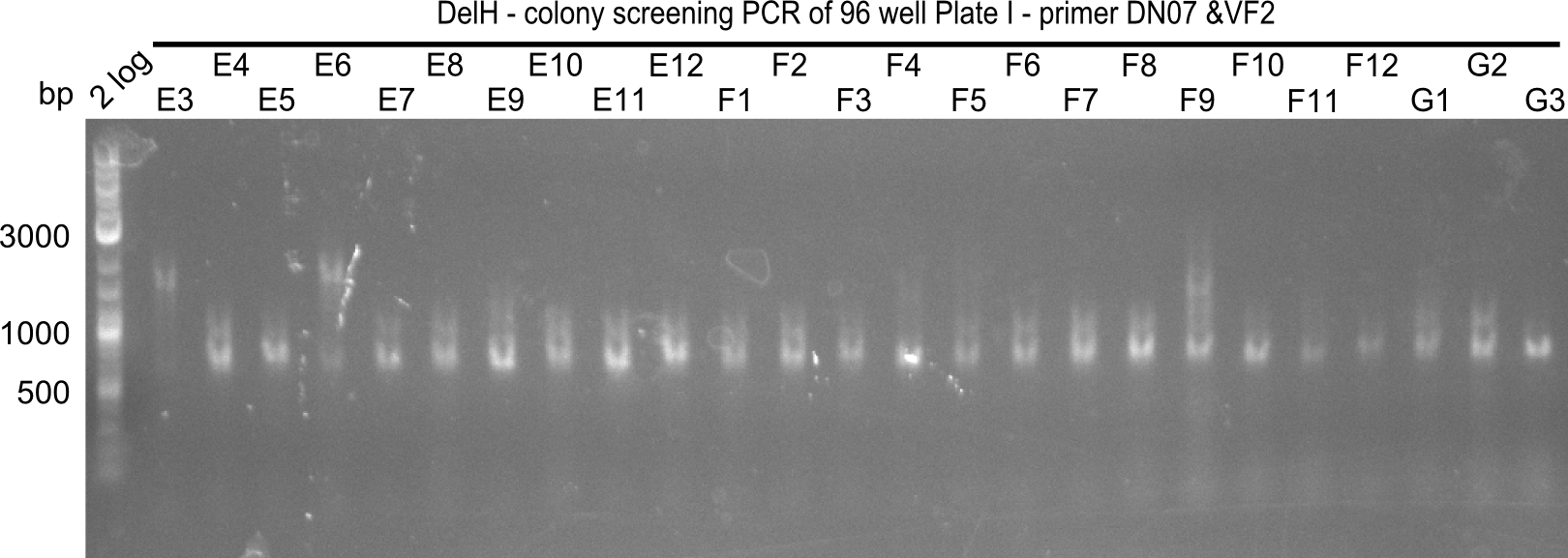
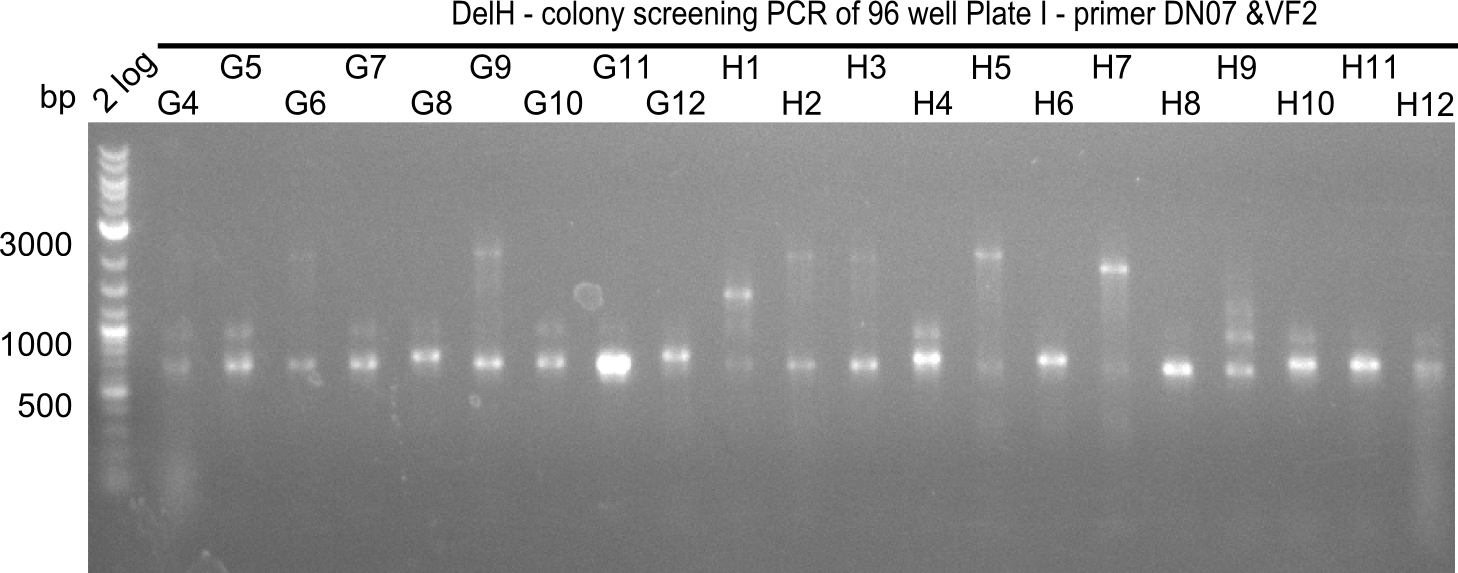
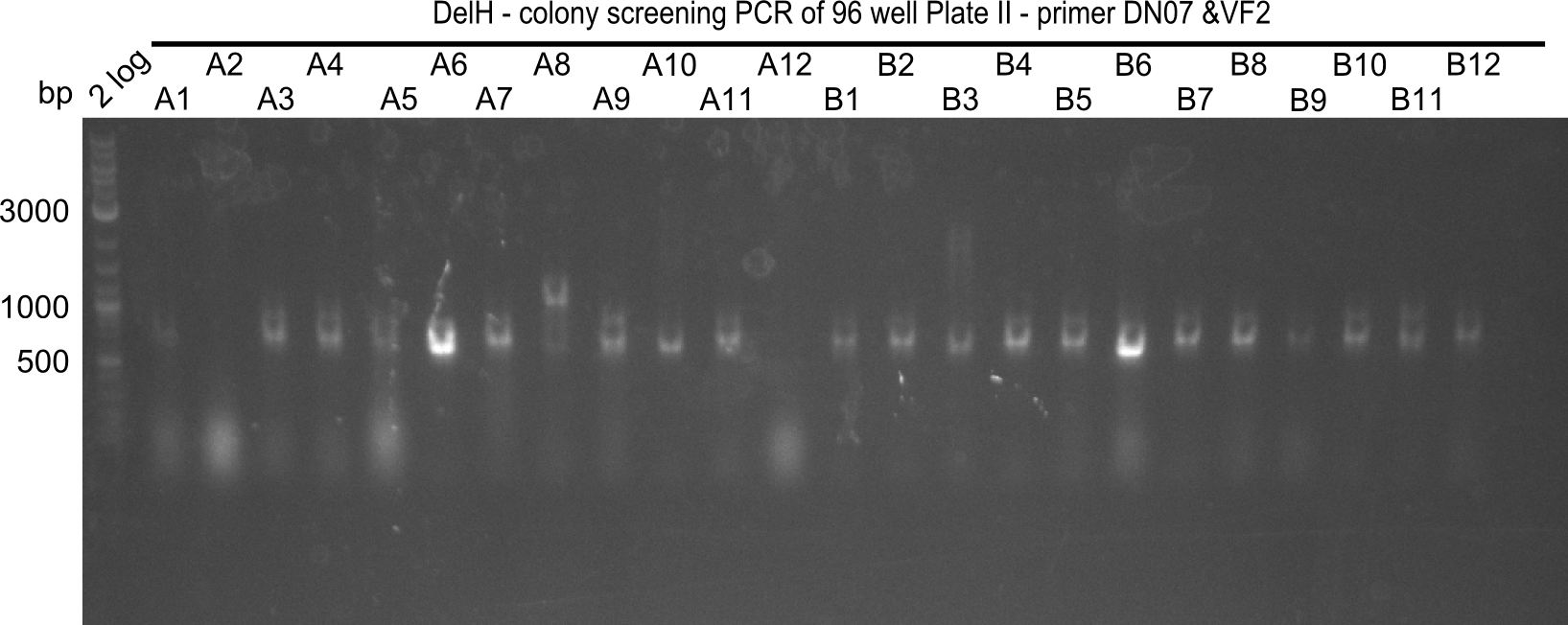
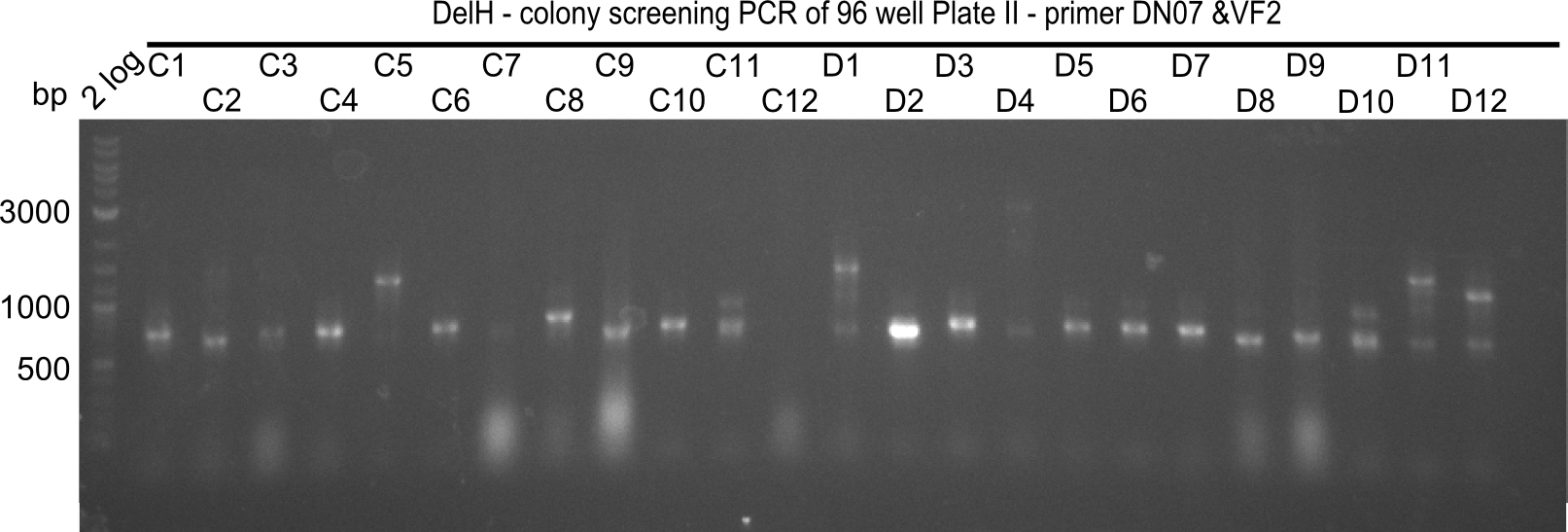
The following colonies seem positive, because they show the expected band at 663 bp
| Picture | Positive colonies |
|---|---|
| 21.12 | - |
| 21.13 | C3, C5, C7, C11, C12, D1, D2, D3, D5, D6, D7, D8, D9, D12 |
| 21.14 | E2, E9, F4, F11 |
| 21.15 | G3, G6, G11, G12, H6, H8, H11 |
Colony-PCR CP.W21.B
| Template | 96x 1 picked colony |
| Expected length [bp] | 663 |
| Named | 1A - 12H, DelH II |
| Primer fw 10 µM | 100x 2 µl VF2 |
| Primer rev 10 µM | 100x 2 µl DN07 |
| One-Taq Polymerase (2x) | 100x 10 µl |
| ddH2O | 96x 6 µl |
| Cycles | Temperature [°C] | Time [s] |
|---|---|---|
| 1 | 95 | 120 |
| 12 | 95 | 30 |
| 68 (touchdown -0.5°C) | 30 | |
| 72 | 45 | |
| 18 | 95 | 30 |
| 65 | 30 | |
| 72 | 45 | |
| 1 | 12 | inf |
Result
Expected band: 663 bp
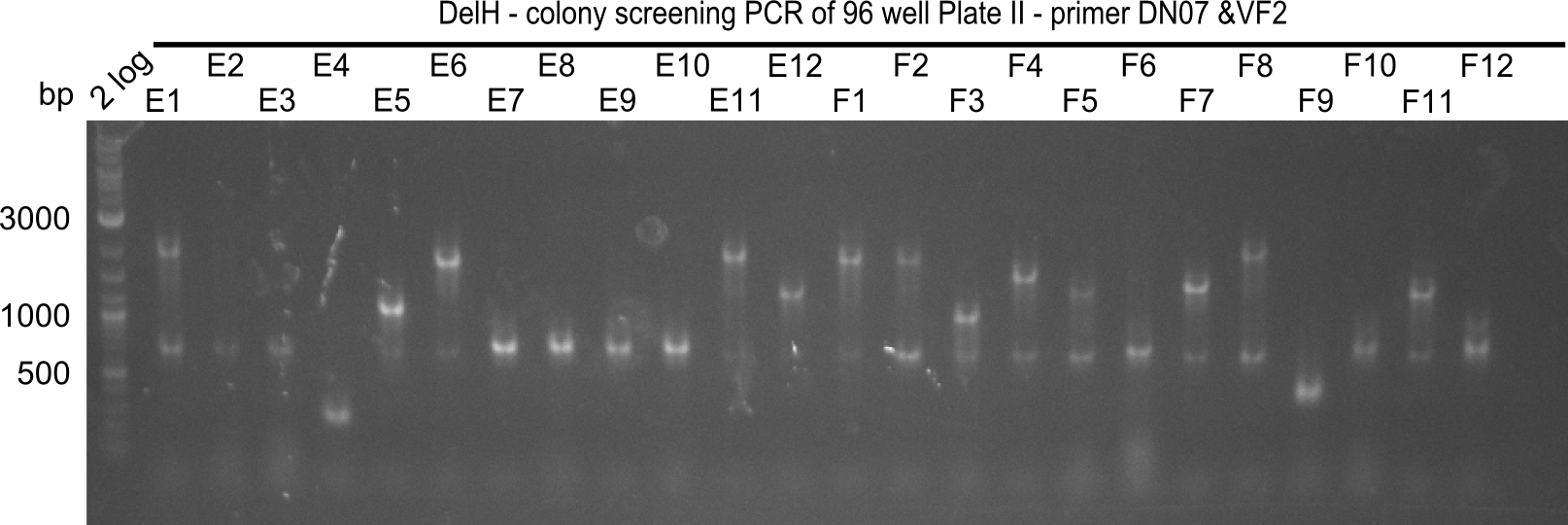
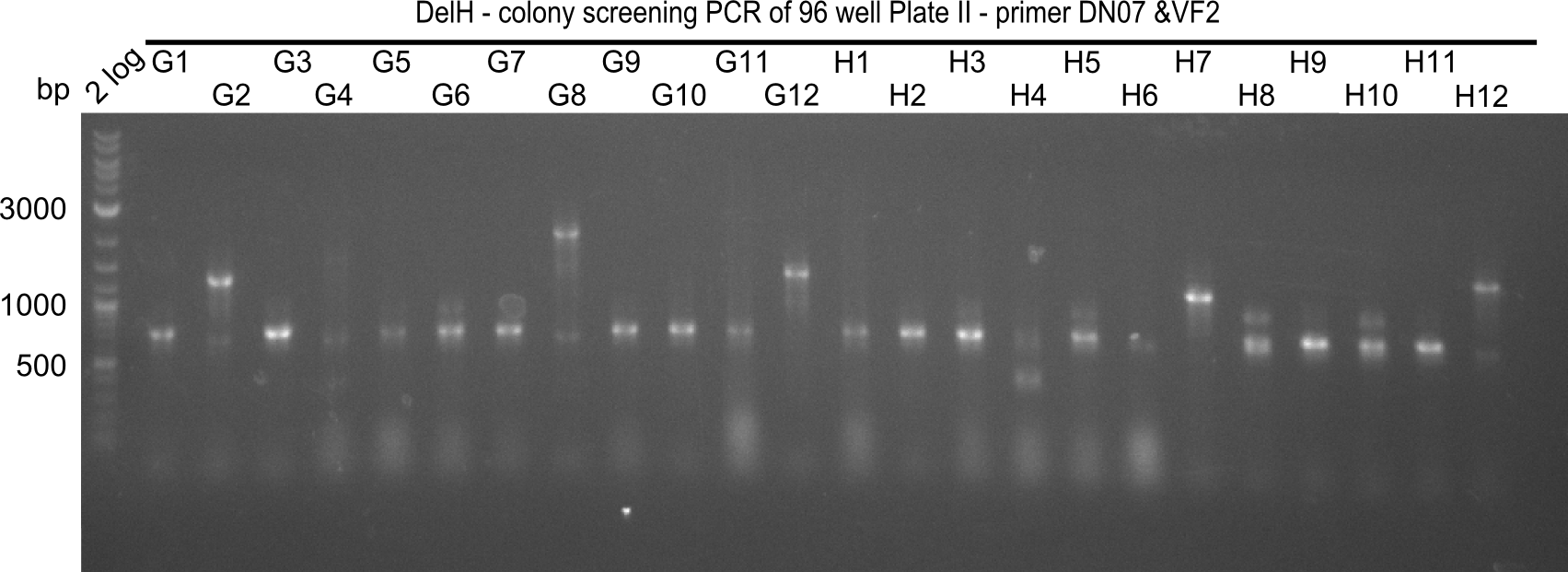
The following colonies seem positive, because they show the expected band at 663 bp
| Picture | Positive colonies |
|---|---|
| 21.16 | A6, A7, A10, B2, B6 |
| 21.17 | C2, C4, C6, C8, C9, C10, D2, D3, D8, D9 |
| 21.18 | E7, E8, E9, E10, F6 |
| 21.19 | G1, G3, G6, G7, G9, G10, H2, H3, H8, H11 |
Test Restriction Digest
The positive screened colonies were digested with PvuI.
| Template DNA | CutSmart Buffer | PvuI | ddH2O | Total Volume |
|---|---|---|---|---|
| 8.5 µl 1 µl | 0.5 µl | - | 10 µl |
Result
Expected bands: 11,621, 8,628 & 2,685 bp
The following colonies show the correct pattern:
| Colonies | Positive in Digest | Concentration in Midiprep [ng/µl] |
|---|---|---|
| I C5 | + | 454 |
| I C7 | + | 1312 |
| I C12 | + | 5995 |
| I G11 | + | 3887 |
| II A6 | + | 1017 |
| II D2 | + | 926 |
Sequencing
Clones C5, C7, C12, G11, II A6 and II D2 were send for sequencing with VF2.
Result
| Colonies | Alignment File |
|---|---|
| I C5 | Sequencing of clone I C5 with DN_07 |
| I C7 | sequencing insufficient |
| I C12 | sequencing insufficient |
| I G11 | discarded |
| II A6 | discarded |
| II D2 | discarded |
Amplification of Backbone
PCR Conditions BB.W21.B
Amplification of backbone parts registry well 2L, transformed 19-09 using HPLC purified HM11
| Reagent | HM11 |
|---|---|
| Template | 1 µl of minipreped pSB6A1 (2L) |
| Primer fw 10 µM | 2 µl HM11 |
| Primer rev 10 µM | 2 µl HM17 |
| Phusion Flash Ready Mix | 10 µl |
| ddH2O | 5 µl |
| Cycles | Temperature [°C] | Time [s] |
|---|---|---|
| 1 | 98 | 5 |
| 12 | 98 | 1 |
| 68 (touchdown -0,5°C) | 5 | |
| 72 | 90 | |
| 18 | 98 | 1 |
| 68 | 5 | |
| 72 | 90 | |
| 1 | 72 | 5 min |
| 1 | 4 | inf |
Result
Expected band: 4.4 Kb
Gel does not show amplified backbone fragment.
- => Further optimize PCR conditions.
PCR Conditions BB.W21.C
| Reagent | HM11 |
|---|---|
| Template | 1 µl of minipreped pSB6A1 (2L) |
| Primer fw 10 µM | 2 µl HM11 |
| Primer rev 10 µM | 2 µl HM17 |
| Phusion Flash Ready Mix | 10 µl |
| ddH2O | 5 µl |
| Cycles | Temperature [°C] | Time [s] |
|---|---|---|
| 1 | 98 | 5 |
| 30 | 98 | 1 |
| 68 | 5 | |
| 72 | 90 | |
| 1 | 72 | 5 min |
| 1 | 4 | inf |
Result
Expected band: ~ 4.4 Kb
Gel shows strong and specific amplification of backbone.
- => Fragments were cut and gel isolated.
Generation of DelH Plasmid pHM04 20-09
Gibson Assembly
| Fragment | Concentration [ng/µl] | Amount with BB16 (FS77) [µl] | Amount with BB16 (HM20) [µl] |
|---|---|---|---|
| DN11-FS66 | 172.5 | 1.77 | 1.84 |
| FS67-FS68 | 120.7 | 2.15 | 2.24 |
| FS69-FS70 | 211.3 | 2.12 | 2.21 |
| FS71-HM08 | 146 | 1.24 | 1.30 |
| BB16 amplified with FS77 | 43.1 | 2.72 | - |
| BB16 amplified with HM20 | 35.8 | - | 2.42 |
- Incubate for 1 h at 50°C
Electroporation
- Afterwards 3 µl of each Gibson assembly were taken and 2 µl ddH2O were added
- With 17 µl of the Gibson assembly, isopropanol purification was performed, DNA was eluted in 20 µl ddH2O
- 6 different electroporations in E.coli DH10ß were performed and grown at RT (see scheme below)
| Electroporation name | Inserted amount of Gibson Assembly | Plated out on agar plates |
|---|---|---|
| HM20 1 | 3 µl (Gibson + ddH2O) | * 10 µl * rest |
| HM20 20 | 20 µl (isopropanol purified) | * 10 µl * rest |
| FS77 1 | 1 µl (Gibson + ddH2O) | * 10 µl * rest |
| FS77 20 | 20 µl (isopropanol purified) | * 10 µl * rest |
New HPLC Purified Primers
As pointed out before, we suspect DelH to be toxic for E. coli and therefore, only colinies containing mutated constructs survive. To avoid this, we ordered new shorter and HPLC purified primers for the assembly of pHM04.
| Identifier | Order date | Note | Sequence |
|---|---|---|---|
| HM_20:BB_HPLC_rev | 11-09-2013 | HPLC version of HM11 Gibson-Primer rev, amplify the Backbone with overlap with the RBS and the lacI-promotor and it creates and overlap to the start of DelH | GATTTGGCGCAGGCGGCCACGGTCCATctagtatttctcctctttc |
| FS_77:BB_HPLC_rev | 11-09-2013 | Gibson-Primer rev, amplify the Backbone with overlap with the RBS and the lacI-promotor and it creates and overlap to the start of DelH | GCGATTTGGCGCAGGCGGCCACGGTCCATCTAGTATTTCTCCTCTTTC |
Mutagenesis of DelH clones I 6B and 15
Strategy
As our clones which have the DelH construct keep on having mutations and deletions we decided to fix this problem by carrying out site-directed mutagenesis. The targeted mutations are the deletion of parts of the ribsome binding site in clone I6B and the deletion at the beginning of DelH in clone 15. For this we make use of two restriction enzymes (MfeI & XbaI) which both only cut in the construct once. By this we excise the fragment with the mutation. The larger fragment (17.3 kbp) without mutation is kept.
Furthermore we ordered four new primers. The smaller fragment is then PCR amplified with those primers in two fragments. Primer HM22 and HM23 cure the mutations. With those primers also recognitions sites for the restriction enzyme BbsI are introduced, which is needed for religation of the two fragments. BbsI cuts two basepair after its recognition site.
HM21 and HM24 are the corresponding primer. They introduce XbaI and MfeI sites as well as in each on BbsI recognition site. Those are needed for religation.
In the end the two fragments are both digested with BbsI. In the adjacent ligation the two fragments can ligate together due to the BbsI recognition sites. Furthermore BbsI opens the introduced MfeI and XbaI sites through which the two fragments can ligate with the digested 17.3 kb fragment.
However the XbaI site is not introduced correctly again. Therefore we can test if the mutagenesis had been successful by digesting with XbaI. If the site is not present anymore we successfully got rid of the mutations.
New Primers
| Identifier | Order date | Note | Sequence |
|---|---|---|---|
| HM21_fw_lacI_BbsI_Xba | 2013-09-15 | Forward primer for cutting out mutated fragment for mutagenesis | TTTTGAAGACAA CTAGGCAATACGCAA |
| HM22_rev_RBS | 2013-09-15 | Reverse Primer in RBS for mutagenesis | TTTTGAAGACAA CTCTTTCTCTAGTATGTGTGAAATTG |
| HM23_fw_RBS | 2013-09-15 | Forward Primer in RBS for mutagenesis | TTTTGAAGACAA AGAGGAGAAATACTAGATGGACCGTGGC |
| HM24_rev_BbsI_MfeI | 2013-09-15 | Reverse primer for cutting out mutated fragment for mutagenesis | TTTTGAAGACAA AATTGGACAGCGCGGCATGCCGGTTG |
Purification of Midiprep of Clone I6B
As can be seen in figure 21.23, the midi-prep of colony I 6B is contaminated. Thus, we tried to purify only the largest band by gel extraction with the QIAEX II Gel Extraction Kit (Qiagen). The procedure was as following:
- Application of 2 µl of midiprep on gel (4596.7 ng/µl --> ~10 µg)
- Excision of band at about 17.4 kb
- Gel extraction of DNA with QIAEX II Gel Extraction Kit (Qiagen)
Result
- The concentration of the purified fragment was only 4.7 ng/µl after extraction.
- => We are trying to elute again.
- The second elution did not work either
Amplification of HM23 to HM24
1x 20 µl for each colony
| Template | 0.2 µl of either midiprep colony I6B or 15 |
|---|---|
| Expected length [kbp] | 5.3 |
| Named | I6B or 15 HM23-HM24 |
| Primer fw 10µM | 2 µl HM23 |
| Primer rev 10 µM | 2 µl HM24 |
| Phusion Flash (2x) | 10 µl |
| ddH2O | 5 µl |
| Cycles | Temperature [°C] | Time [s] |
|---|---|---|
| 1 | 98 | 10 s |
| 12 | 98 | 1 |
| 68 (touchdown -0.5°C) | 5 | |
| 72 | 1:50 min | |
| 18 | 98 | 1 |
| 65 | 5 | |
| 72 | 1:50 min | |
| 1 | 72 | 7 min |
| 1 | 12 | inf |
| Cycles | Temperature [°C] | Time [s] |
|---|---|---|
| 1 | 98 | 10 |
| 30 | 98 | 1 |
| 69 | 5 | |
| 72 | 1:50 min | |
| 1 | 72 | 7 min |
| 1 | 12 | inf |
| Template | 0.2 µl of either midiprep colony I6B or 15 |
|---|---|
| Expected length [kbp] | 5.3 |
| Named | I6B or 15 HM23-HM24 |
| Primer fw 10µM | 2 µl HM23 |
| Primer rev 10 µM | 2 µl HM24 |
| Phusion Flash (2x) | 10 µl |
| ddH2O | 4 µl |
| DMSO | 1 µl |
| Cycles | Temperature [°C] | Time [s] |
|---|---|---|
| 1 | 98 | 10 s |
| 30 | 98 | 1 s |
| 72 | 1:40 min | |
| 1 | 72 | 7 min |
| 1 | 12 | inf |
Result
Gels for amplification of HM23 to HM24
Amplification of HM21 to HM22
| Template | 0.2 µl of either midiprep colony I6B or 15 |
|---|---|
| Expected length [kbp] | 200 bp |
| Named | I6B or 15 HM21-HM22 |
| Primer fw 10µM | 2 µl HM21 |
| Primer rev 10µM | 2 µl HM22 |
| Phusion Flash (2x) | 10 µl |
| ddH2O | 5 µl |
| Cycles | Temperature [°C] | Time [s] |
|---|---|---|
| 1 | 98 | 10 |
| 30 | 98 | 1 |
| 63 | 5 | |
| 72 | 10 | |
| 1 | 72 | 5 min |
| 1 | 12 | inf |
Result
Gels for amplification of HM21 to HM22
Restriction Digest of pHM04 (I6B) with XbaI and MfeI
| Reagent | Volume [µl] |
|---|---|
| Midiprep colony I6B or 15 | 0.5 |
| Cutsmart buffer | 2 |
| XbaI | 1.5 |
| MfeI-HF | 1.5 |
| ddH2O | 14.5 |
- Incubation for 2 h at 37°C
Result
- Expected band pattern: 17.3 kbp, 5.3 kbp
Gels for restriction Digest of pHM04 (I6B) with XbaI and MfeI
- The MfeI already expired in 2011
- => We will test digest the construct again with a new enzyme
Restriction Digest of pHM04 (I6B) with XbaI and MfeI
| Reagent | Volume [µl] |
|---|---|
| Midiprep colony I6B or 15 | 0.5 |
| Cutsmart buffer | 5 |
| XbaI | 1.5 |
| MfeI-HF | 1.5 |
| ddH2O | 14.5 |
| Incubation for 2 h at 37°C | Expected band pattern: 17.3 kbp, 5.3 kbp |
| Reagent | Volume [µl] |
|---|---|
| Midiprep colony I6B purified 1:10 | 1 |
| Cutsmart buffer | 2 |
| XbaI | 1 |
| MfeI-HF | 1 |
| ddH2O | 15 |
| Incubation at room temperature, over night | Expected band pattern: 17.3 kbp, 5.3 kbp |
Result
Due to the contamination in DelH it was not possible to decide whether the fragment really had been digested. Thus we have to test whether MfeI Cutting site is really present.
Test for MfeI Cutting Site
| Reagent | Volume [µl] |
|---|---|
| Midiprep colony I6B | 0.5 |
| Cutsmart buffer | 2 |
| NotI-HF | 1 |
| ddH2O | 16.5 |
| Reagent | Volume [µl] |
|---|---|
| Midiprep colony I6B | 0.5 |
| Cutsmart buffer | 2 |
| NotI-HF | 1 |
| MfeI-HF | 1 |
| ddH2O | 15.5 |
Incubation at room temperature over night.
--> As the upper band is lower for the digest with both enzymes the MfeI restriction site is apparently present. Therefore the mutagenesis is tried.
Restriction Digest of Fragment HM21-HM22 (I6B) and HM23-HM24 (I6B) with BbsI
PCR fragments: HM21-HM22 (36.6 ng/µl) and HM23-HM24 (32.4 ng/µl)
| Reagent | Volume [µl] |
|---|---|
| PCR fragment | 20 |
| 2.1 buffer | 5 |
| BbsI | 1 |
| ddH2O | 24 |
Ligation of Digested pHM04 (I6B), HM21-HM22 and HM23-HM24
| Reagent | Volume [µl] |
|---|---|
| digested and purified pHM04 (I6B) | 3 |
| digested HM21-HM22 | 0.5 |
| digested HM23-HM24 | 0.5 |
| T4 ligase buffer | 2 |
| T4 ligase | 1 |
| ddH2O | 13 |
Transformation of Ligation
1 µl and 5 µl were transformed in 50 µl electrocompetent DH10β each by electroporation. The cells were recovered in 400 µl SOC media and plated on LB Amp.
 "
"