Team:Heidelberg/Templates/Del week10 G
From 2013.igem.org
Contents |
05-07-2013
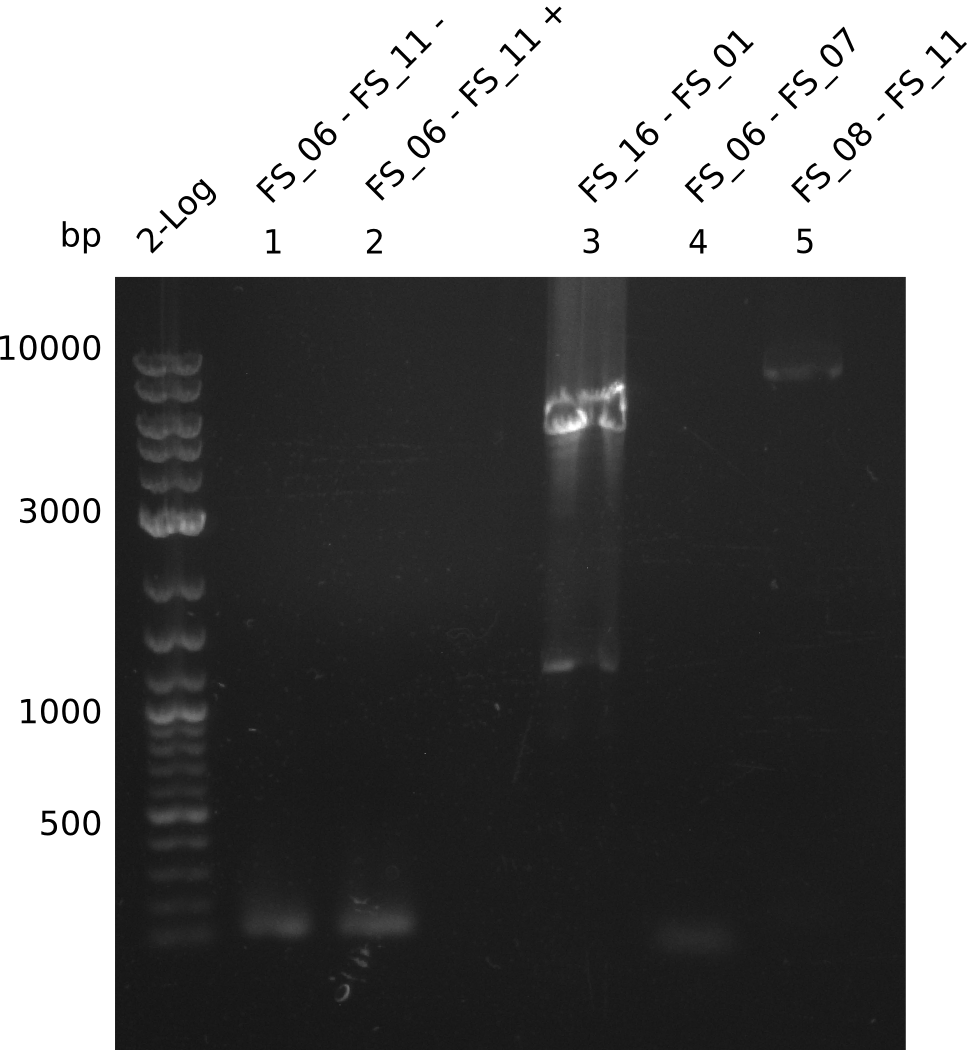
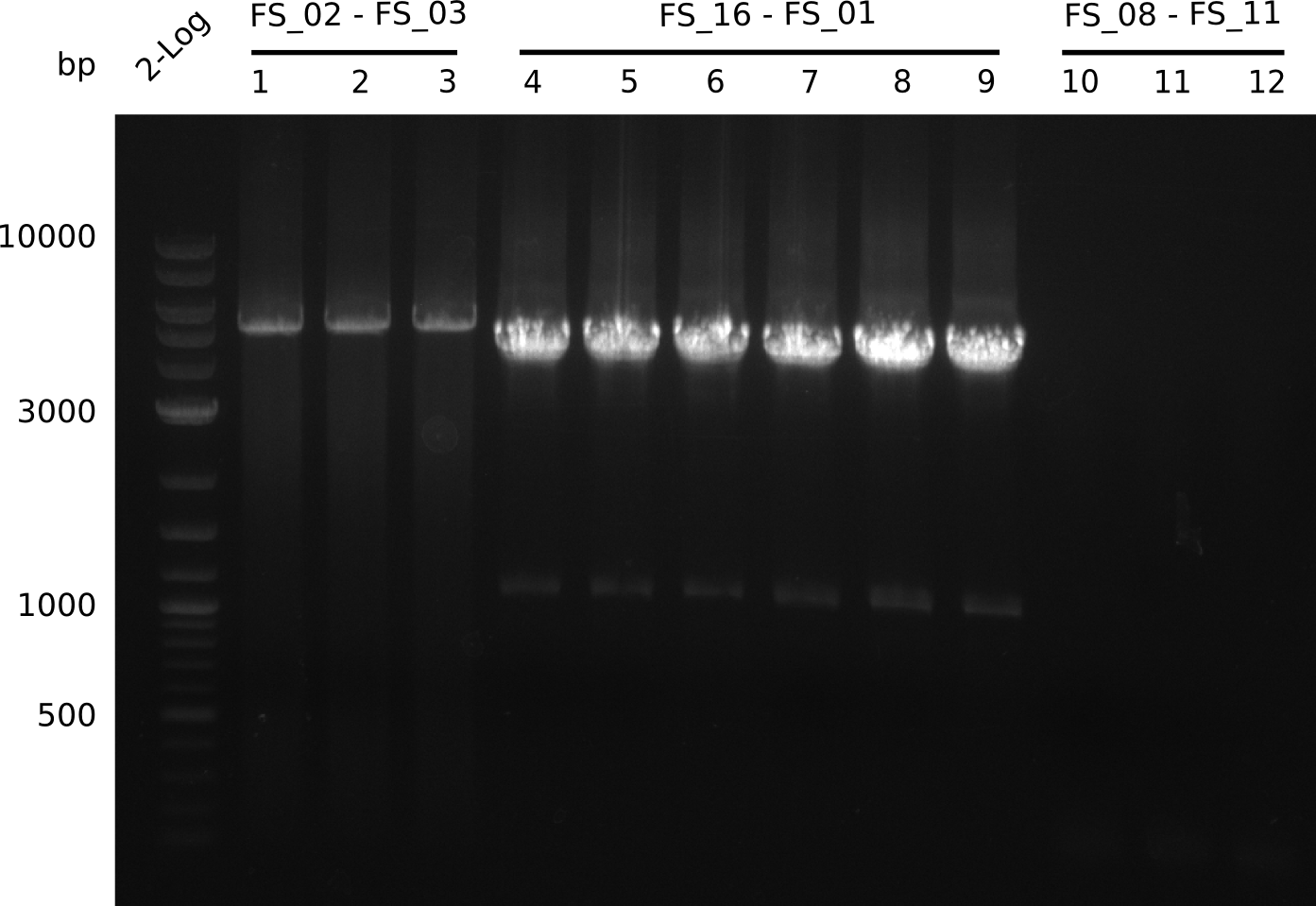
Amplification from FS_08 to FS_11; 6.5 kb
- Reaction
| what | µl |
|---|---|
| D. acidovorans DSM-39 | 1 |
| FS_08: (1/10) | 1 |
| FS_11: (1/10) | 1 |
| Phusion Master Mix | 10 |
| dd H2O | 6 |
| DMSO | 1 |
- Conditions
| Biorad MyCycler | ||
|---|---|---|
| Cycles-PCR | temperature [°C] | Time [s] |
| 1 | 98 | 5 |
| 14 | 98 | 1 |
| 62 ↓ 0.5 | 5 | |
| 72 | 3:20 min | |
| 16 | 98 | 1 |
| 60 | 5 | |
| 72 | 3:20 min | |
| 1 | 72 | 12 min |
| 1 | 4 | inf |
Results:
- A weak band was visible, but it was on the wrong height.
- Accidently the band was cut anyway.
- Either the primers did not bind or the DNA still had to many secondary structures --> the consequence is to change the annealing temperature.
07-07-2013
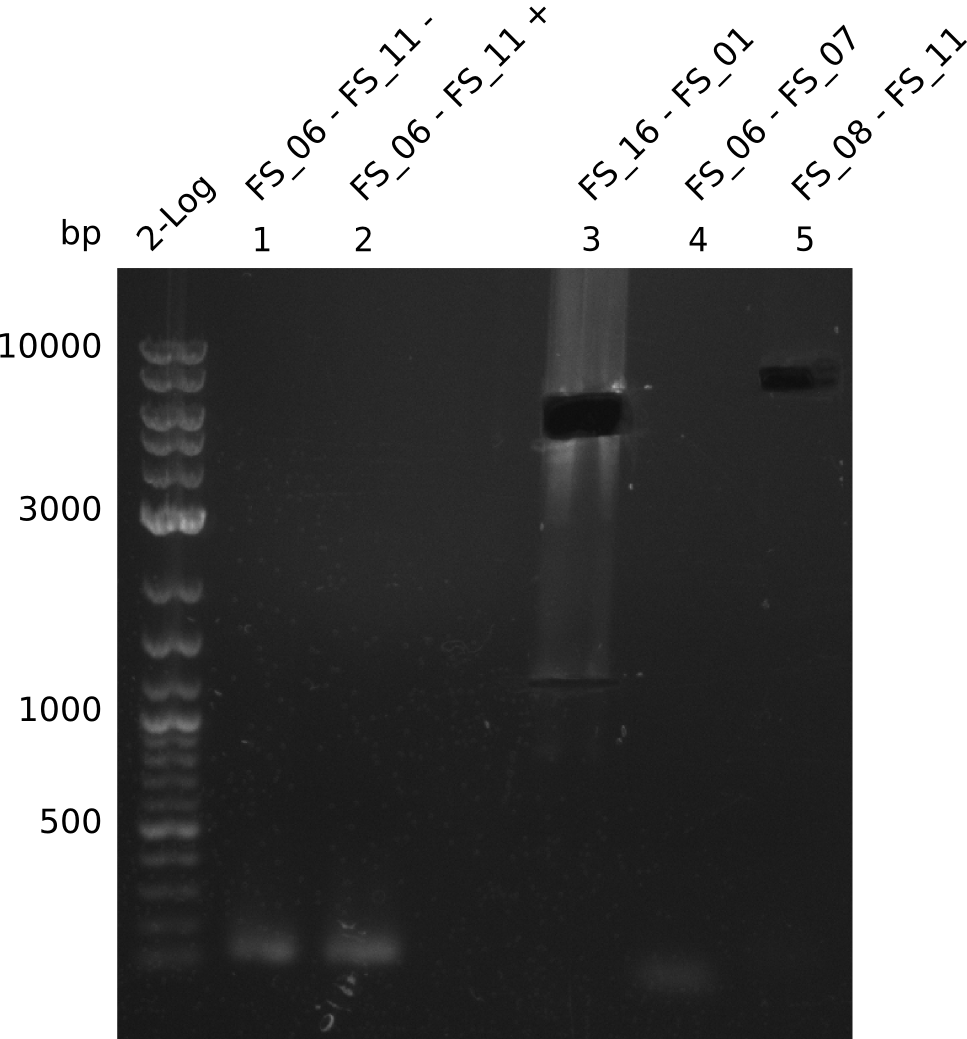
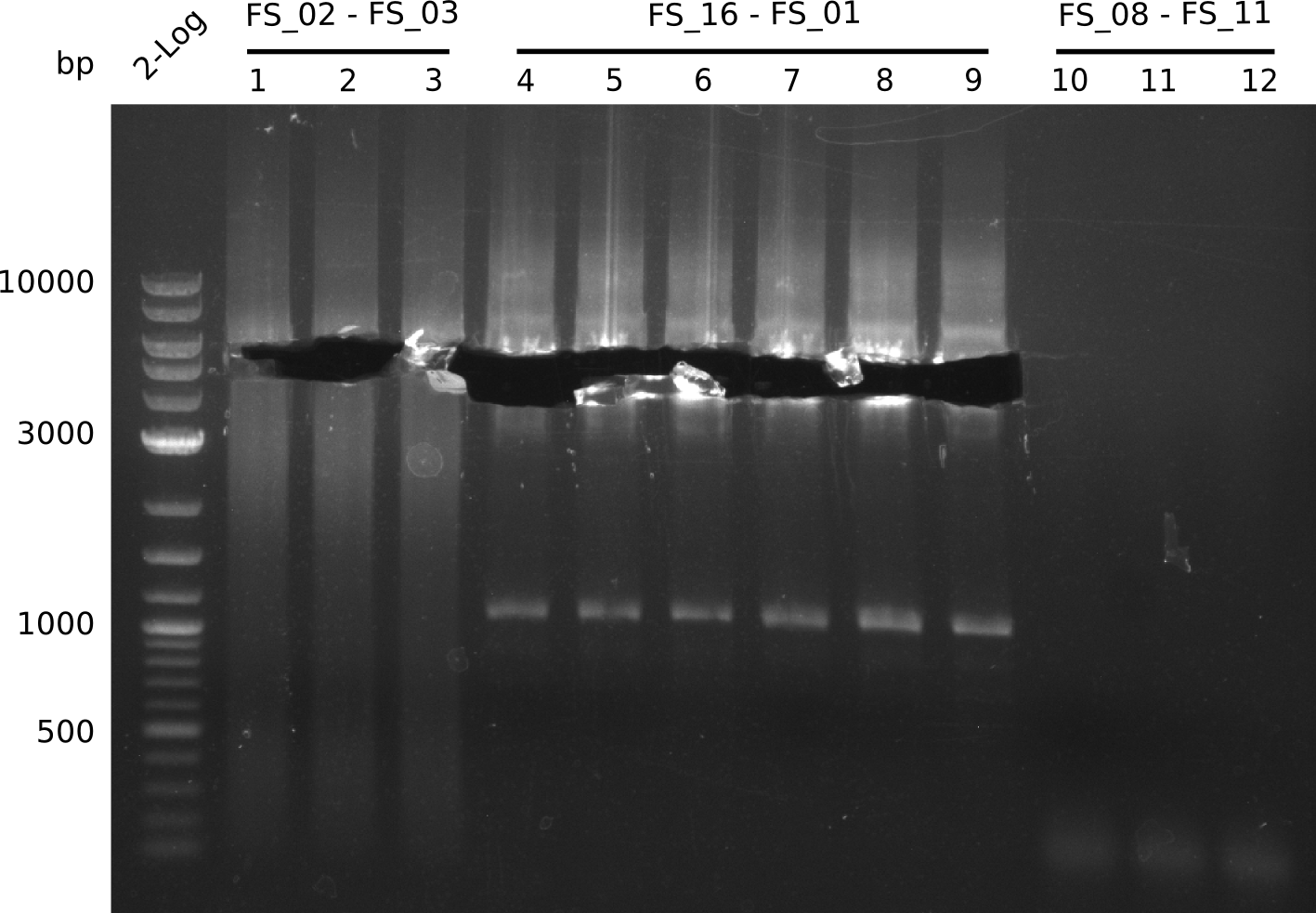
Amplification from FS_08 to FS_11; 6.5 kb
- Reaction
| what | µl |
|---|---|
| D. acidovorans DSM-39 | 1 |
| FS_08: (1/10) | 2.5 |
| FS_11: (1/10) | 2.5 |
| Phusion Master Mix | 25 |
| dd H2O | 19 |
| DMSO | 2.5 |
- Conditions
| Biorad MyCycler | ||
|---|---|---|
| Cycles-PCR | temperature [°C] | Time [s] |
| 1 | 98 | 5 |
| 12 | 98 | 1 |
| 66 ↓ 0.5 | 5 | |
| 72 | 2:30 min | |
| 18 | 98 | 1 |
| 63 | 5 | |
| 72 | 2:30 min | |
| 1 | 72 | 10 min |
| 1 | 4 | inf |
Results:
- There was no band visible on the gel.
- Either the primers did not bind or the DNA still had to many secondary structures --> the consequence is to change the annealing temperature.
 "
"