Team:Heidelberg/Templates/Del week11 EG
From 2013.igem.org
(Difference between revisions)
| Line 2: | Line 2: | ||
===Amplification from FS_04 to FS_07; 11.1 kb=== | ===Amplification from FS_04 to FS_07; 11.1 kb=== | ||
| - | + | ||
| - | + | ||
:'''Reaction''' | :'''Reaction''' | ||
{| class="wikitable" | {| class="wikitable" | ||
| Line 78: | Line 77: | ||
| - | [[File: | + | [[File:Heidelberg_20130712 DelLP DelFG DelEG DelEG(PHII).png|150px|thumb|PCR for amplification of DelLP, DelFG and DelEG; lane1=Ladder log2, lane2=delLP(11.07), lane3=DelFG(11.07), lane4=DelEG (Phusion flash, 11.07), lane5=DelEG (Phusion II,11.07), lane6=1kb ladder plus; DelLP=6.4kbp, DelFG=5.3kbp, DelEG=16.4kbp; run at 100 V, 0.8 % gel (TAE)]] |
:'''Reaction''' | :'''Reaction''' | ||
| Line 130: | Line 129: | ||
===Amplification from FS_04 to FS_11; 17.5 kb; Phusion II=== | ===Amplification from FS_04 to FS_11; 17.5 kb; Phusion II=== | ||
| - | [[File: | + | [[File:Heidelberg_20130712 DelLP DelFG DelEG DelEG(PHII).png|150px|thumb|PCR for amplification of DelLP, DelFG and DelEG; lane1=Ladder log2, lane2=delLP(11.07), lane3=DelFG(11.07), lane4=DelEG (Phusion flash, 11.07), lane5=DelEG (Phusion II,11.07), lane6=1kb ladder plus; DelLP=6.4kbp, DelFG=5.3kbp, DelEG=16.4kbp; run at 100 V, 0.8 % gel (TAE)]] |
:'''Reaction''' | :'''Reaction''' | ||
{| class="wikitable" | {| class="wikitable" | ||
| Line 186: | Line 185: | ||
===Amplification from FS_04 to FS_07; 11.1 kb=== | ===Amplification from FS_04 to FS_07; 11.1 kb=== | ||
| - | [[File: | + | [[File:Heidelberg_20130714 FS04 TO FS07.png |150px|thumb|PCR for amplification of DelEG (FS04-FS07, 14.07); lane1=log2 Marker, lane2=DelEG (protocol 68touchdown), lane3=DelEG (protocol 72touchdown); desired amplicon size=11kbp run at 100 V, 0.8 % gel (TAE)]] |
| - | [[File: | + | [[File:Heidelberg_20130714 FS04 TO FS07 cut.png |150px|thumb|PCR for amplification of DelEG (FS04-FS07, 14.07) after excision; run at 100 V, 0.8 % gel (TAE)]] |
:'''Reaction''' | :'''Reaction''' | ||
Revision as of 02:02, 30 September 2013
Contents |
09-07-2013
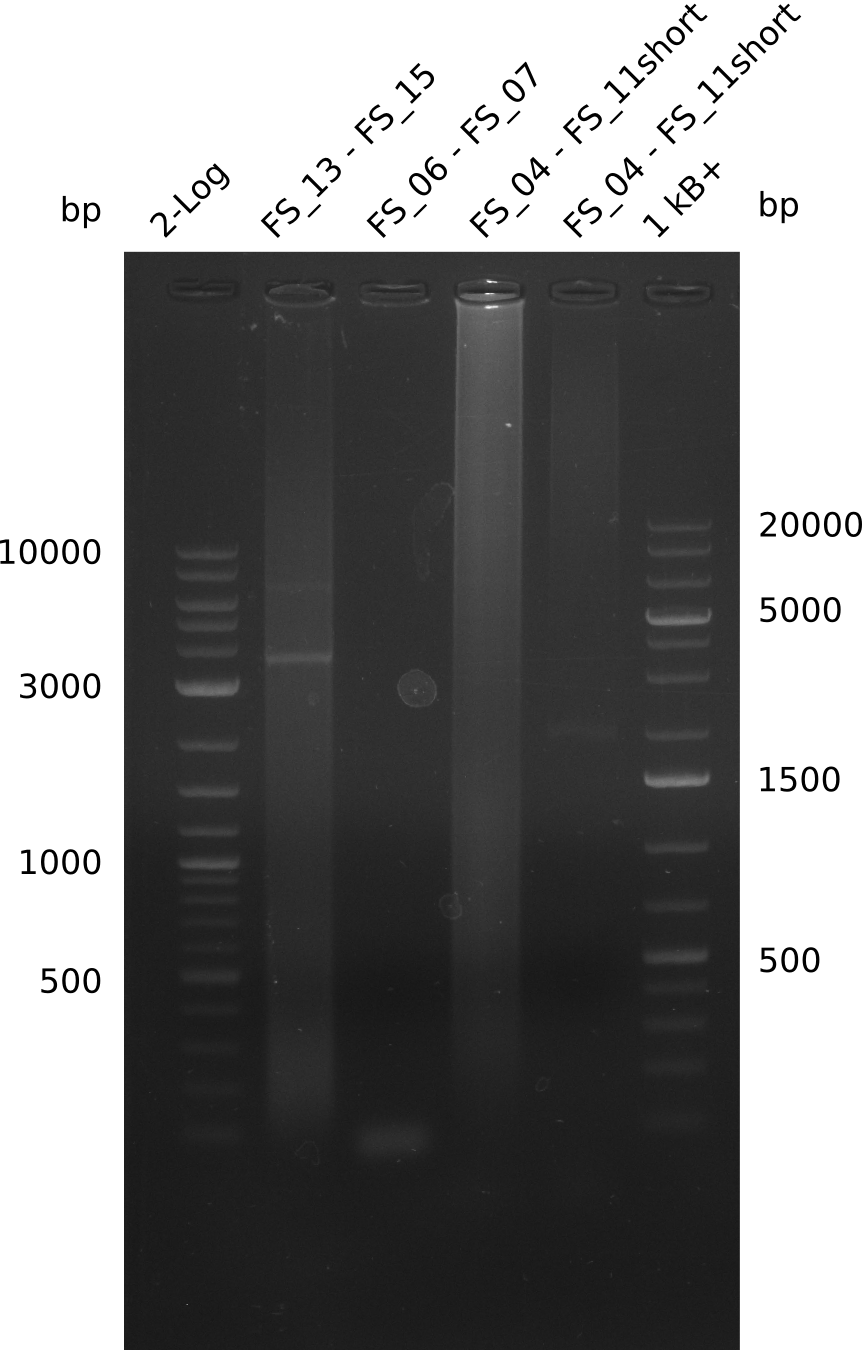
Amplification from FS_04 to FS_07; 11.1 kb
- Reaction
| what | µl |
|---|---|
| D. acidovorans DSM-39 | 1 |
| FS_04: (1/10) | 1 |
| FS_07: (1/10) | 1 |
| Phusion Master Mix | 10 |
| DMSO | 1 |
| dd H2O | 6 |
- Conditions I
| Cycles-PCR | temperature [°C] | Time [s] |
|---|---|---|
| 1 | 98 | 10 |
| 12 | 98 | 1 |
| 68 ↓ 0.5 | 5 | |
| 72 | 3 min | |
| 18 | 98 | 1 |
| 66 | 5 | |
| 72 | 3 min | |
| 1 | 72 | 10 min |
| 1 | 4 | inf |
- Conditions II
| Biorad MyCycler | ||
|---|---|---|
| Cycles-PCR | temperature [°C] | Time [s] |
| 1 | 98 | 10 |
| 5 | 98 | 1 |
| 68 | 5 | |
| 72 | 3 min | |
| 25 | 98 | 1 |
| 72 | 5 | |
| 72 | 3 min | |
| 1 | 72 | 10 min |
| 1 | 4 | inf |
Results:
- Amplification of DelEG did not work
11-07-2013
Amplification from FS_04 to FS_11s; 17.5 kb
- Reaction
| what | µl |
|---|---|
| D. acidovorans DSM-39 | 1 |
| FS_04: (1/10) | 1 |
| FS_11_short: (1/10) | 1 |
| Phusion flash Master Mix | 10 |
| DMSO | 1 |
| dd H2O | 6 |
- Conditions
| Biorad MyCycler | ||
|---|---|---|
| Cycles | temperature [°C] | Time [s] |
| 1 | 98 | 10 |
| 12 | 98 | 1 |
| 68 ↓ 0.5 | 5 | |
| 72 | 5:30 | |
| 18 | 98 | 1 |
| 66 | 5 | |
| 72 | 5:30 | |
| 1 | 72 | 15min |
| 1 | 12 | inf |
Results:
- Amplification of DelEG did not work
- Experiment will be repeated with NEB Phusion II Polymerase as Phusion II is not provided as mastermix and therefore GC-buffer can be used
Amplification from FS_04 to FS_11; 17.5 kb; Phusion II
- Reaction
| what | µl |
|---|---|
| D. acidovorans DSM-39 | 1 |
| FS_04: (1/10) | 1 |
| FS_11_short: (1/10) | 1 |
| Phusion II | 0.2 |
| DNTP | 0.4 |
| Buffer | 4 |
| DMSO | 0.6 |
| dd H2O | 11.8 |
- Conditions
| Biorad MyCycler | ||
|---|---|---|
| Cycles | temperature [°C] | Time [s] |
| 1 | 98 | 30 |
| 12 | 98 | 5 |
| 68 ↓ 0.5 | 30 | |
| 72 | 8:10 | |
| 18 | 98 | 5 |
| 66 | 30 | |
| 72 | 8:10 | |
| 1 | 72 | 15min |
| 1 | 17 | inf |
Results:
- Amplification of DelEG did not work
- it seems not to be possible to amplify the desired 17 kbp fragent with the chosen primers and the given template, primercombination will be changed in further amplification attempts
14-07-2013
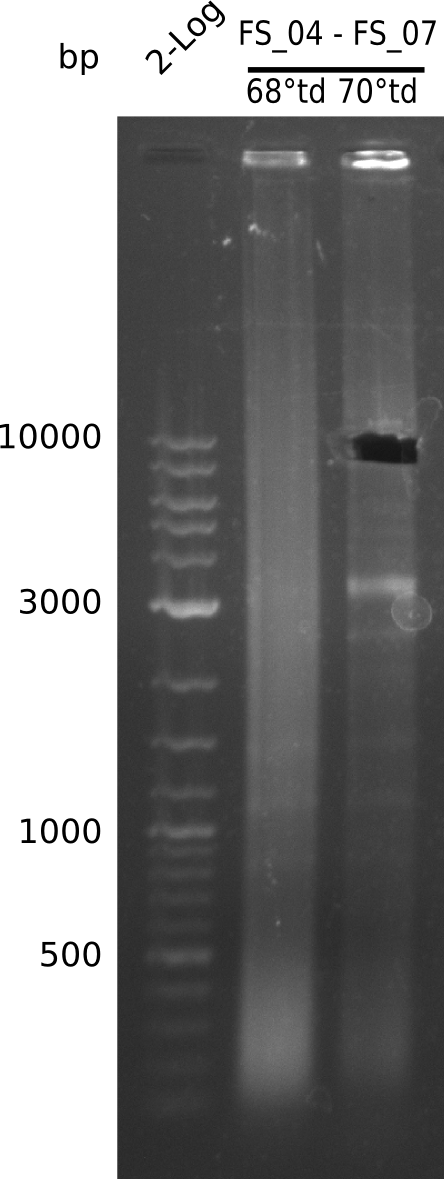
Amplification from FS_04 to FS_07; 11.1 kb
- Reaction
| what | µl |
|---|---|
| D. acidovorans DSM-39 | 1 |
| FS_04: (1/10) | 2 |
| FS_07: (1/10) | 2 |
| Phusion flash Master Mix | 10 |
| DMSO | 1 |
| dd H2O | 4 |
- Conditions I
Cycler incubation room right
| Biorad MyCycler | ||
|---|---|---|
| Cycles | temperature [°C] | Time [s] |
| 1 | 98 | 10 |
| 12 | 98 | 1 |
| 68 ↓ 0.5 | 5 | |
| 72 | 3:00 min | |
| 18 | 98 | 1 |
| 66 | 5 | |
| 72 | 3:00 min | |
| 1 | 72 | 10 min |
| 1 | 12 | inf |
- Conditions II
Cycler incubation room left
| Biorad MyCycler | ||
|---|---|---|
| Cycles | temperature [°C] | Time [s] |
| 1 | 98 | 10 |
| 12 | 98 | 1 |
| 70 ↓ 0.5 | 5 | |
| 72 | 3:00 min | |
| 18 | 98 | 1 |
| 68 | 5 | |
| 72 | 3:00 min | |
| 1 | 72 | 10 min |
| 1 | 12 | inf |
Results:
- Amplification of DelEG worked with a touchdown PCR starting from 70°C annealing temperature
- band was cut out and DNA purified using QIAquick Gel Extraction Kit
- PCR will be repeated to increase the amount of DNA and gather the concentrations necessary for Gibson Assembly
 "
"