Team:Heidelberg/Templates/Del week12 FG
From 2013.igem.org
(Difference between revisions)
(→Re-Amplification from FS_06 to FS_09; 8.5 kb; 13-07-2013)) |
|||
| Line 2: | Line 2: | ||
==15-07-2013== | ==15-07-2013== | ||
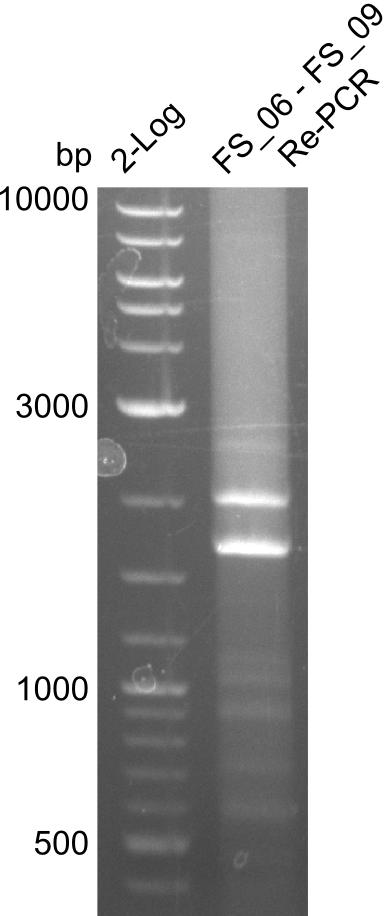
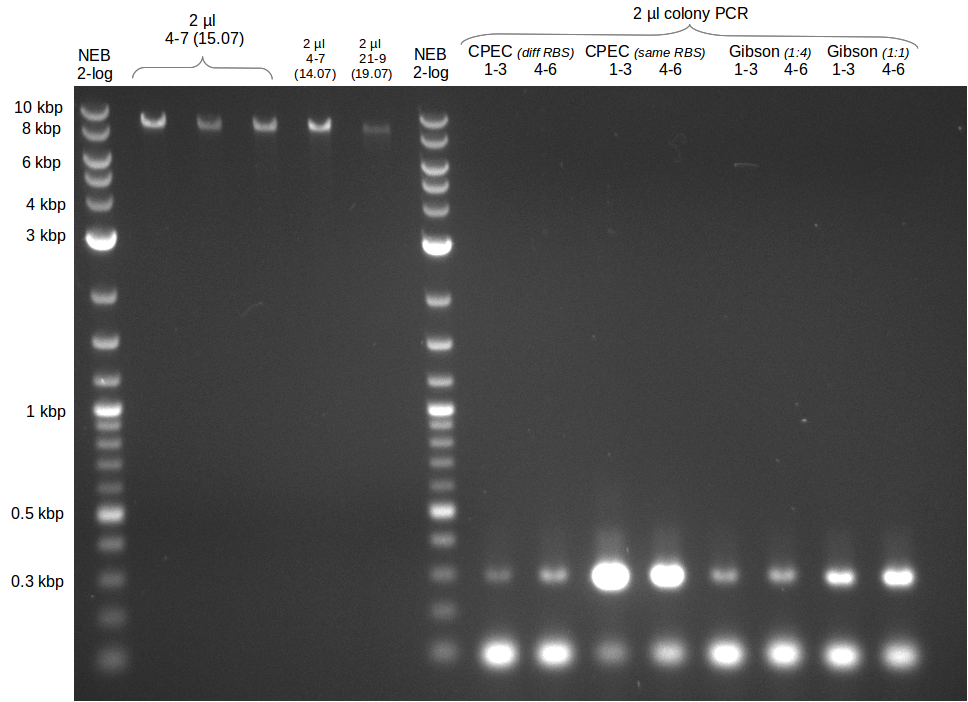
[[File:Heidelberg_20130715 FS06 TO FS09 reamplifikation.png|150px|thumb|Re-PCR for amplification of DelFG (15.07) run at 100 V, 0.8 % gel (TAE)]] | [[File:Heidelberg_20130715 FS06 TO FS09 reamplifikation.png|150px|thumb|Re-PCR for amplification of DelFG (15.07) run at 100 V, 0.8 % gel (TAE)]] | ||
| - | ===Re-Amplification from FS_06 to FS_09; 8.5 kb; | + | ===Re-Amplification from FS_06 to FS_09; 8.5 kb; 13-07-2013)=== |
:'''Reaction''' | :'''Reaction''' | ||
| Line 9: | Line 9: | ||
! what !! µl | ! what !! µl | ||
|- | |- | ||
| - | | DelEG ( | + | | DelEG (FS_06-FS_09; 13-07-2013) || 1 |
|- | |- | ||
| FS_06: (1/10) || 2 | | FS_06: (1/10) || 2 | ||
Revision as of 18:23, 3 October 2013
Contents |
15-07-2013
Re-Amplification from FS_06 to FS_09; 8.5 kb; 13-07-2013)
- Reaction
| what | µl |
|---|---|
| DelEG (FS_06-FS_09; 13-07-2013) | 1 |
| FS_06: (1/10) | 2 |
| FS_09: (1/10) | 2 |
| Phusion flash Master Mix | 10 |
| DMSO | 1 |
| dd H2O | 4 |
- Conditions
Cycler incubation room right
| Biorad MyCycler | ||
|---|---|---|
| Cycles | temperature [°C] | Time [s] |
| 1 | 98 | 10 |
| 30 | 98 | 1 |
| 72 | 3:00 min | |
| 1 | 72 | 10 min |
| 1 | 12 | inf |
Results:
- Amplification of DelFG did not work
- two unecpected bands appeared like in the previous trials
19-07-2013
Amplification from FS_20 to FS_09; 8.5 kb
- Reaction
| what | µl |
|---|---|
| D. acidovorans DSM-39 | 1 |
| FS_20: (1/10) | 2 |
| FS_09: (1/10) | 2 |
| Phusion flash Master Mix | 10 |
| DMSO | 1 |
| dd H2O | 4 |
- Conditions
| Biorad T100 | ||
|---|---|---|
| Cycles | temperature [°C] | Time [s] |
| 1 | 98 | 10 |
| 12 | 98 | 1 |
| 68 ↓ 0.5 | 5 | |
| 72 | 2:50 | |
| 18 | 98 | 1 |
| 66 | 5 | |
| 72 | 2:50 | |
| 1 | 72 | 10min |
| 1 | 12 | inf |
Results:
- Amplification of DelFG did not work with FS_20 to FS_09
- as PCR worked with a different set of Primers beeing FS_21 to FS_09, see below, the PCR for this primer combination will be optimized instead of using FS_20 to FS_09
Amplification from FS_21 to FS_09; 8.5 kb
- Reaction
| what | µl |
|---|---|
| D. acidovorans DSM-39 | 1 |
| FS_21: (1/10) | 2 |
| FS_09: (1/10) | 2 |
| Phusion flash Master Mix | 10 |
| DMSO | 1 |
| dd H2O | 4 |
- Conditions
-->New cycler (not two block)
| Biorad T100 | ||
|---|---|---|
| Cycles | temperature [°C] | Time [s] |
| 1 | 98 | 10 |
| 12 | 98 | 1 |
| 68 ↓ 0.5 | 5 | |
| 72 | 2:50 | |
| 18 | 98 | 1 |
| 66 | 5 | |
| 72 | 2:50 | |
| 1 | 72 | 10min |
| 1 | 12 | inf |
Results:
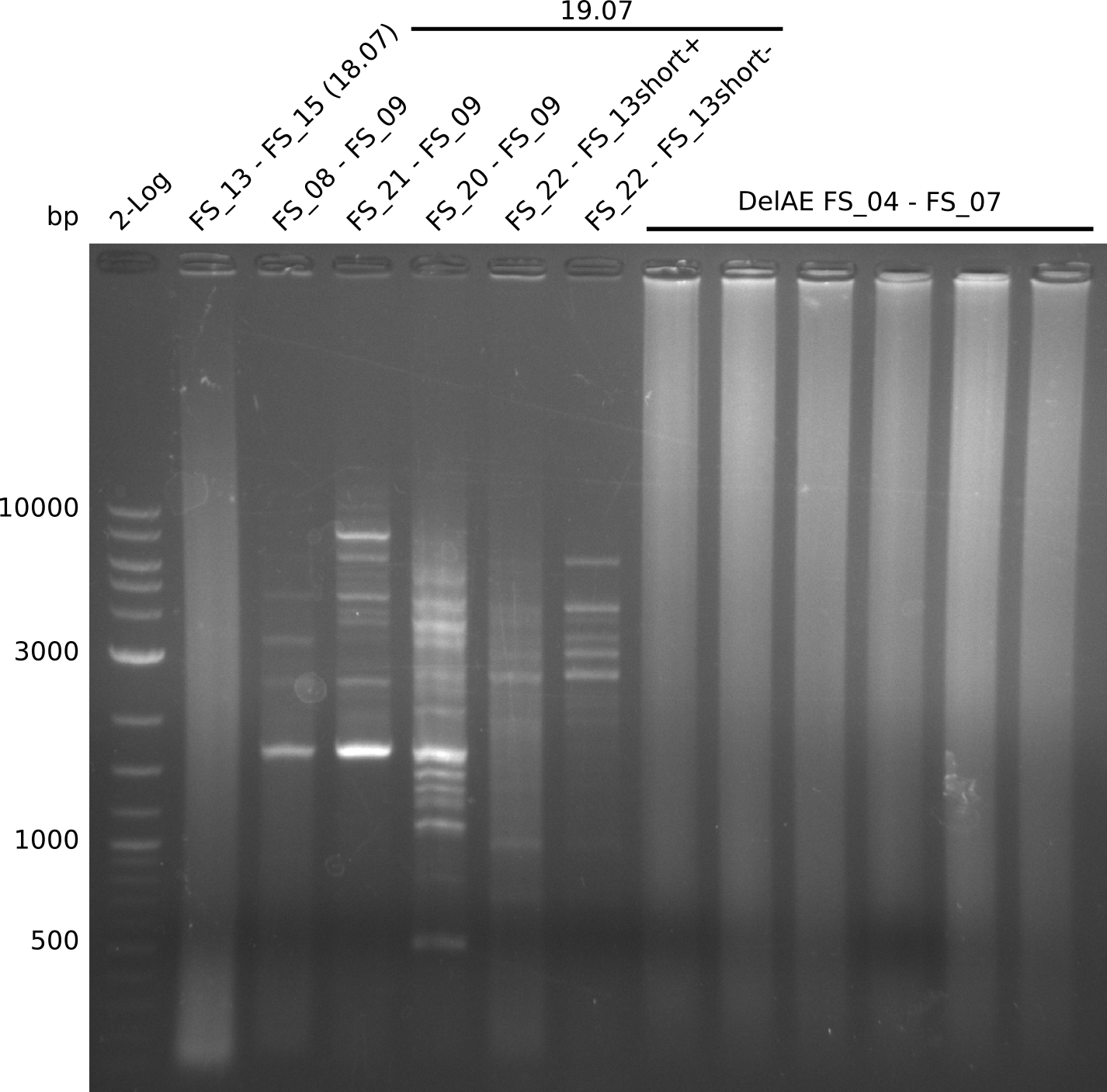
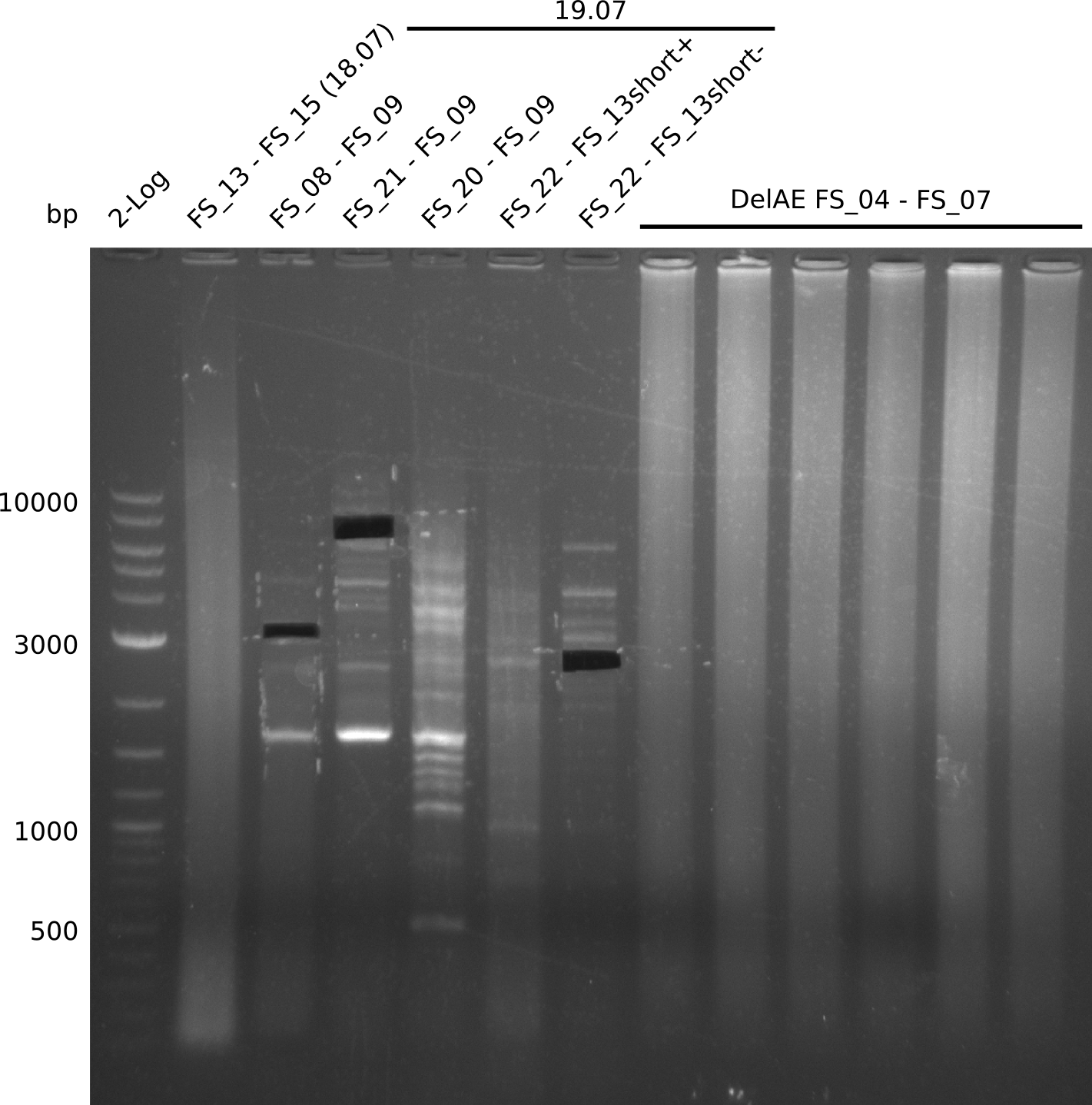
- Amplification worked with FS_21 to FS_09 but not with FS_20 to FS_09
- band was cut out and DNA purified using QIAquick Gel Extraction Kit
- PCR will be repeated to get rid of side product at about 1.5 kb as well as smear, therefore annealing temperature will be increased
20-07-2013
Amplification from FS_21 to FS_09; 8.5 kb
- Reaction
| what | µl |
|---|---|
| D. acidovorans DSM-39 | 1 |
| FS_21: (1/10) | 2 |
| FS_09: (1/10) | 2 |
| Phusion flash Master Mix | 10 |
| DMSO | 1 |
| dd H2O | 4 |
- Conditions
| Biorad C1000 Touch Block A | ||
|---|---|---|
| Cycles | temperature [°C] | Time [s] |
| 1 | 98 | 10 |
| 12 | 98 | 1 |
| 70 ↓ 0.5 | 5 | |
| 72 | 2:50 | |
| 18 | 98 | 1 |
| 68 | 5 | |
| 72 | 2:50 | |
| 1 | 72 | 10min |
| 1 | 12 | inf |
Results:
- Amplification of DelFG did not work only an uneqxpected band a smear occured, but the intended product was not detectable
- other primer combinations might be tried or PCR has to be repeated with the previous conditions not leading to an optimal product quality
21-07-2013
Amplification from FS_20 to FS_07; 5.2 kb
2x 20µl (with, without DMSO)
- Reaction
| what | µl |
|---|---|
| D. acidovorans DSM-39 | 1 |
| FS_20: (1/10) | 2 |
| FS_07: (1/10) | 2 |
| Phusion flash Master Mix | 10 |
| DMSO | 1/- |
| dd H2O | 4/5 |
- Conditions
| Biorad C1000 Block A | ||
|---|---|---|
| Cycles | temperature [°C] | Time [s] |
| 1 | 98 | 5 |
| 12 | 98 | 1 |
| 68 ↓ 0.5 | 5 | |
| 72 | 2:00 | |
| 18 | 98 | 1 |
| 63 | 5 | |
| 72 | 2:00 | |
| 1 | 72 | 10min |
| 1 | 12 | inf |
Results:
- Amplification of DelFG did not work perfectly, neither with nor without 5% DMSO, nevertheless band of expected size was cut out carefully to be used for a Re-PCR
Re-PCR from FS_21 to FS_09; 8.1kb; 19-07-2013)
- Reaction
| what | µl |
|---|---|
| Gel extracted fragments FS_21 to FS_09 (19-07-2013) | 2 |
| FS_21: (1/10) | 2 |
| FS_09: (1/10) | 2 |
| Phusion flash Master Mix | 10 |
| DMSO | 1 |
| dd H2O | 3 |
- Conditions
| Biorad T100 | ||
|---|---|---|
| Cycles | temperature [°C] | Time [s] |
| 1 | 98 | 10 |
| 12 | 98 | 1 |
| 68 ↓ 0.5 | 5 | |
| 72 | 2:50 | |
| 18 | 98 | 1 |
| 66 | 5 | |
| 72 | 2:50 | |
| 1 | 72 | 10min |
| 1 | 12 | inf |
Results:
- Amplification of DelFG did not work with primers FS_21 to FS_09
- PCR will be repeated with different primers
 "
"