Team:Heidelberg/Templates/Del week15 AF
From 2013.igem.org
(Difference between revisions)
(Created page with "==07-08-2013== ===Amplification I from FS_02 to FS_05; 11.2 kb=== [[File:20130807 2log FS2-5(AF) FS21-26(FG) FS8-23(G)besch.png|150px|thumb|Amplifcation of DelAF(I), DelFG, DelG ...") |
|||
| Line 1: | Line 1: | ||
==07-08-2013== | ==07-08-2013== | ||
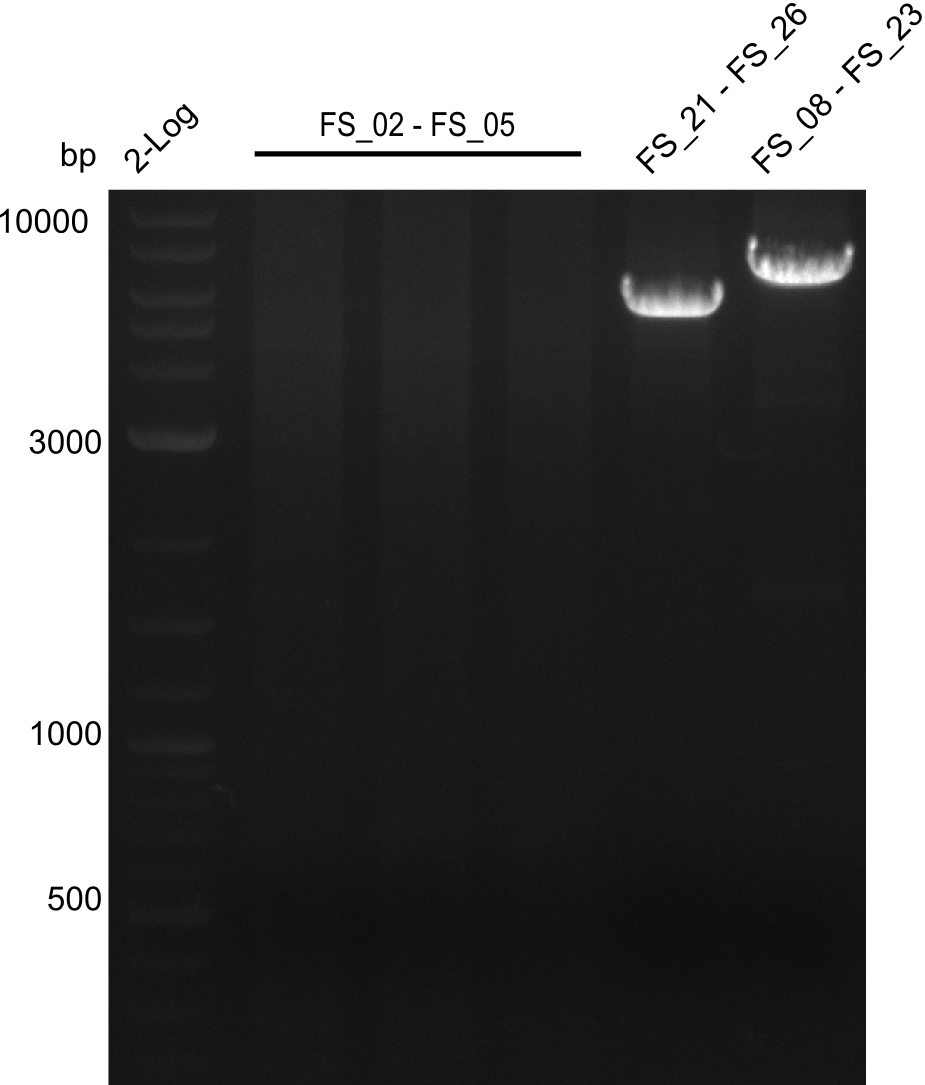
===Amplification I from FS_02 to FS_05; 11.2 kb=== | ===Amplification I from FS_02 to FS_05; 11.2 kb=== | ||
| - | [[File: | + | [[File:Heidelberg_20130807 2log FS2-5(AF) FS21-26(FG) FS8-23(G)besch.png|150px|thumb|Amplifcation of DelAF(I), DelFG, DelG (07.08); run at 100 V, 0.8 % gel (TAE)]] |
| - | [[File: | + | [[File:Heidelberg_20130807 2log FS2-5(AF) FS21-26(FG) FS8-23(G)cut.png|150px|thumb||Amplifcation of DelAF(I), DelFG, DelG (07.08), cut]] |
| Line 54: | Line 54: | ||
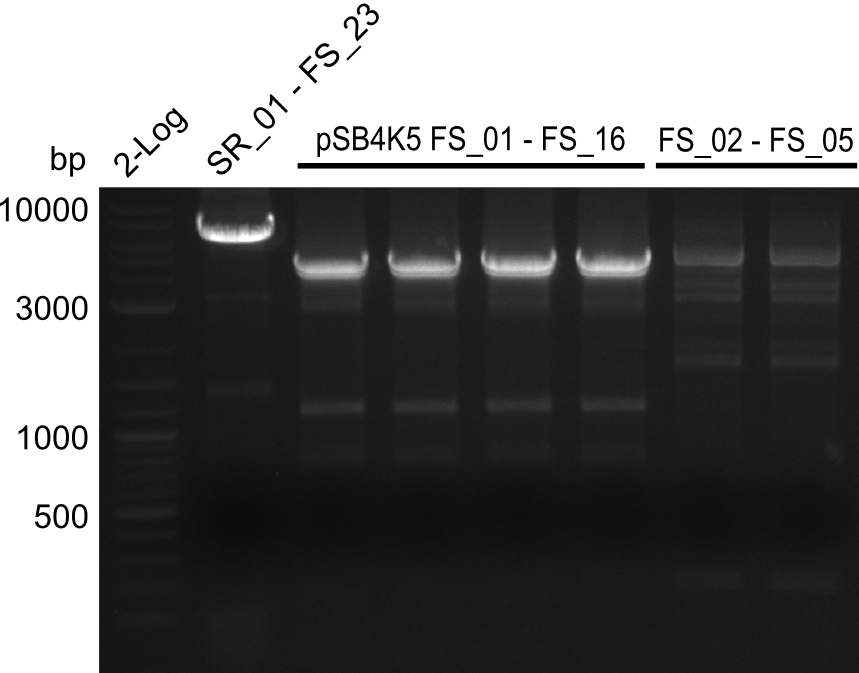
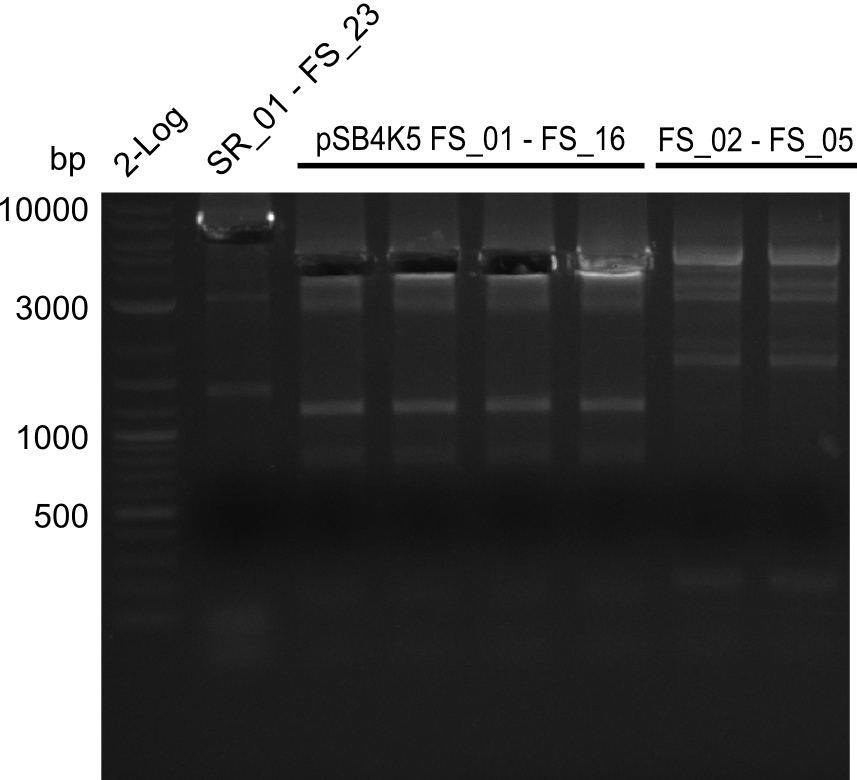
===Amplification II from FS_02 to FS_05; 11.2 kb=== | ===Amplification II from FS_02 to FS_05; 11.2 kb=== | ||
| - | [[File: | + | [[File:Heidelberg_20130807 log2 SR01-FS23(G) 4xpSB4K5 2xFS02-FS05(AF)besch.png|150px|thumb|Amplifcation of DelG (SR01-FS23), pSB4K5 and DelAFII; run at 100 V, 0.8 % gel (TAE)(07.08)]] |
| - | [[File: | + | [[File:Heidelberg_20130807 log2 SR01-FS23(G) 4xpSB4K5 2xFS02-FS05(AF) cut.png|150px|thumb|Amplifcation of DelG (SR01-FS23), pSB4K5 and DelAFII cut; run at 100 V, 0.8 % gel (TAE)(07.08)]] |
:'''Reaction''' | :'''Reaction''' | ||
| Line 108: | Line 108: | ||
==08-08-2013== | ==08-08-2013== | ||
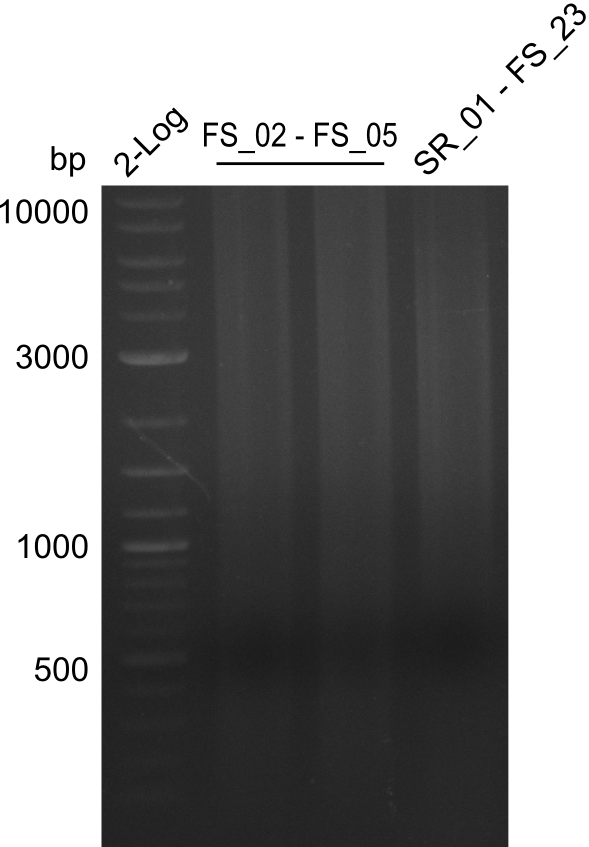
===Amplification from FS_02 to FS_05; 11.2 kb=== | ===Amplification from FS_02 to FS_05; 11.2 kb=== | ||
| - | [[File: | + | [[File:Heidelberg_20130809 2log 2xFS02-FS05 SR01-FS23.png|150px|thumb|Amplifcation of DelAF and DelG; run at 100 V, 0.8 % gel (TAE)(07.08)]] |
| Line 163: | Line 163: | ||
===Amplification from FS_02 to FS_05; 11.2 kb=== | ===Amplification from FS_02 to FS_05; 11.2 kb=== | ||
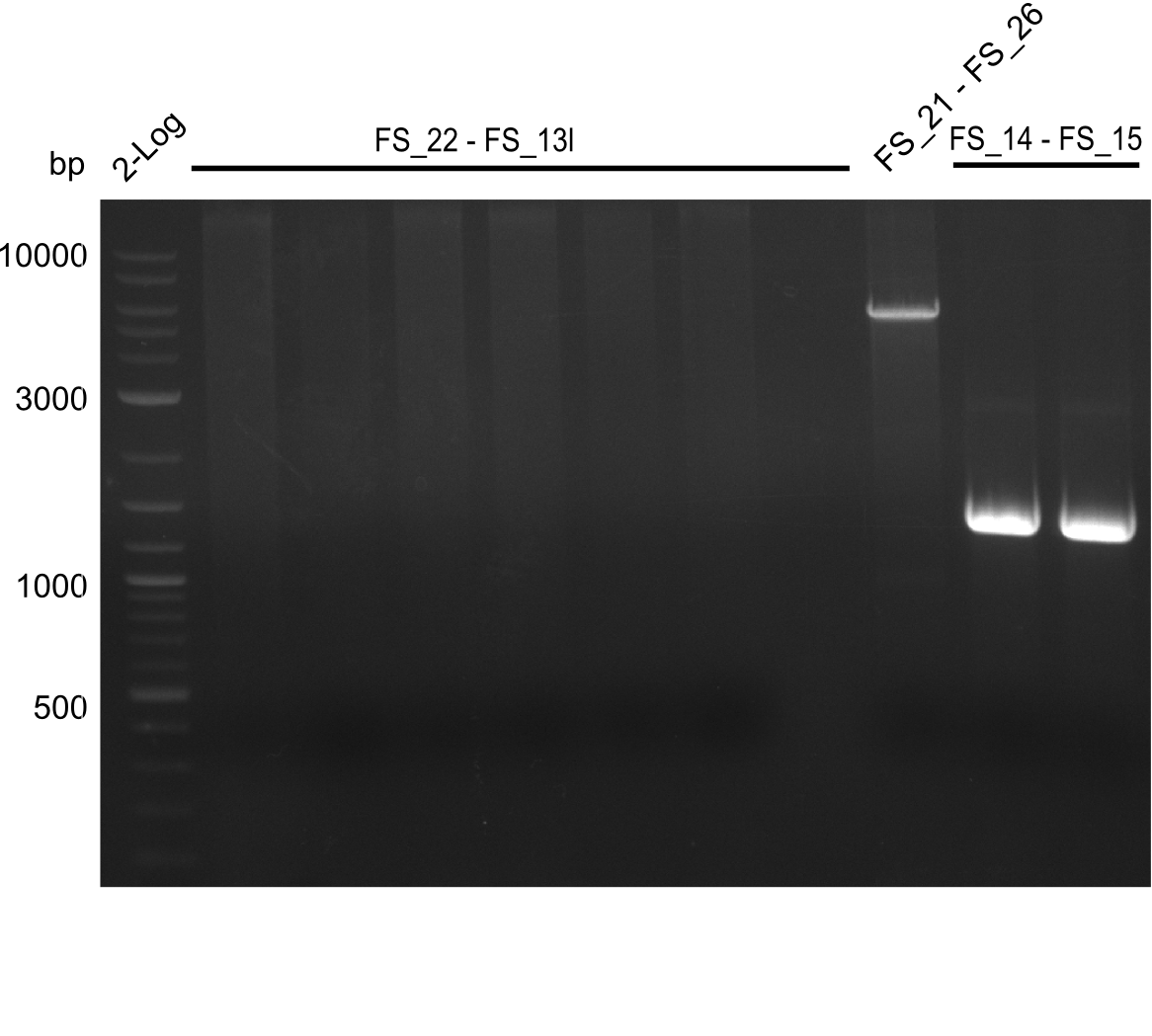
| - | [[File: | + | [[File:Heidelberg_20130809 2log 4xOPfromagarplate FS21-FS26 2xL 2log.png|150px|thumb|Amplifcation of DelOP (I), DelFG and DelL ; run at 100 V, 0.8 % gel (TAE)(09.08)]] |
| - | [[File: | + | [[File:Heidelberg_20130809 2log 4xOPfromagarplate FS21-FS26 2xL cut.png|150px|thumb|Amplifcation of DelOP (I), DelFG and DelL ; run at 100 V, 0.8 % gel (TAE)(09.08)]] |
| Line 222: | Line 222: | ||
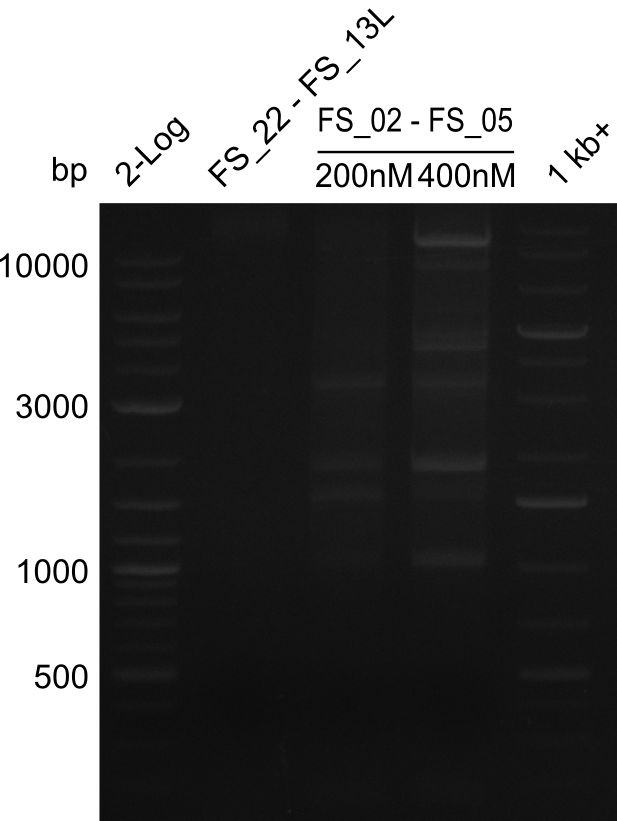
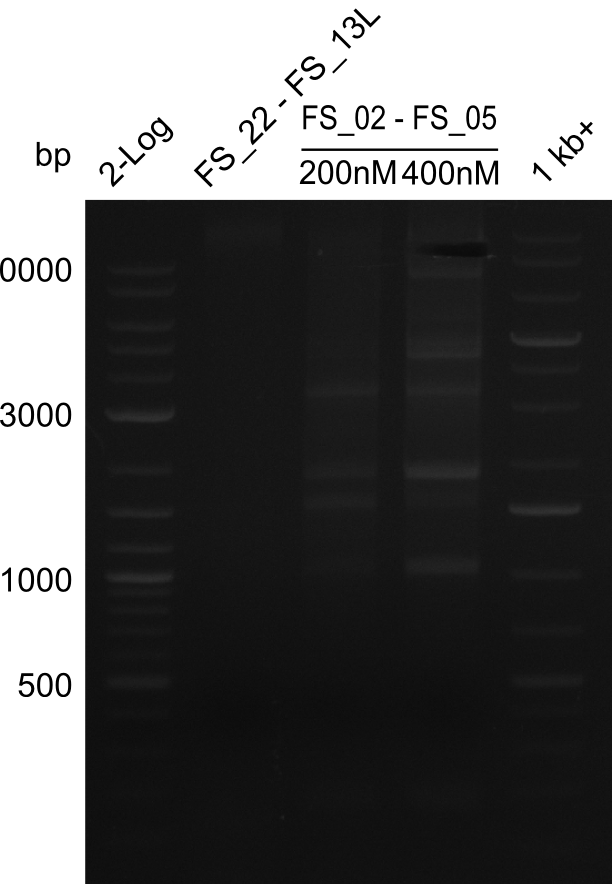
===Amplification from FS_02 to FS_05; 11.2 kb=== | ===Amplification from FS_02 to FS_05; 11.2 kb=== | ||
| - | [[File: | + | [[File:Heidelberg_20130810 2log FS22-13l FS02-05(AF)200nM FS02-05(AF)400nM 1kbruler.png|150px|thumb|Amplifcation of DelOP and DelAF ; run at 135 V, 0.8 % gel (TAE)(10.08)]] |
| - | [[File: | + | [[File:Heidelberg_20130810 2log FS22-13l FS02-05(AF)200nM FS02-05(AF)400nM 1kbruler cut.png|150px|thumb|Amplifcation of DelOP and DelAF after cutting ; run at 135 V, 0.8 % gel (TAE)(10.08)]] |
| Line 273: | Line 273: | ||
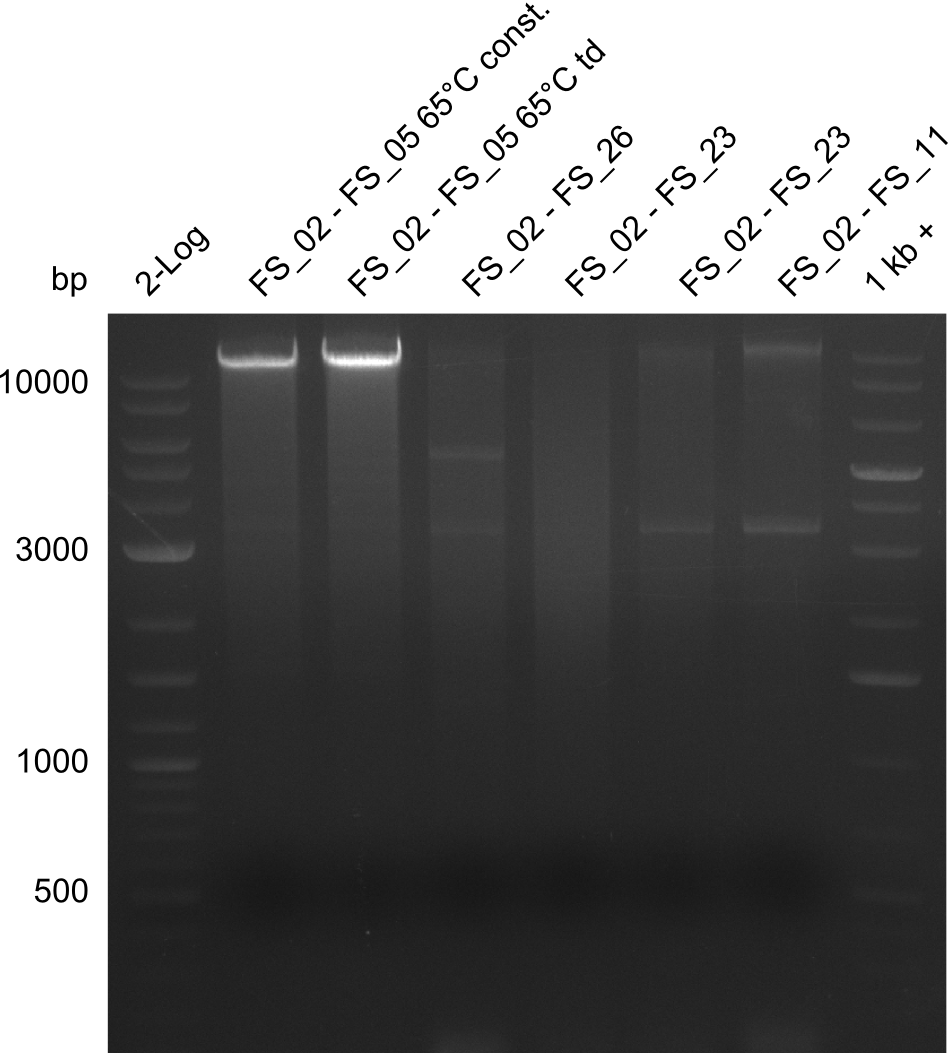
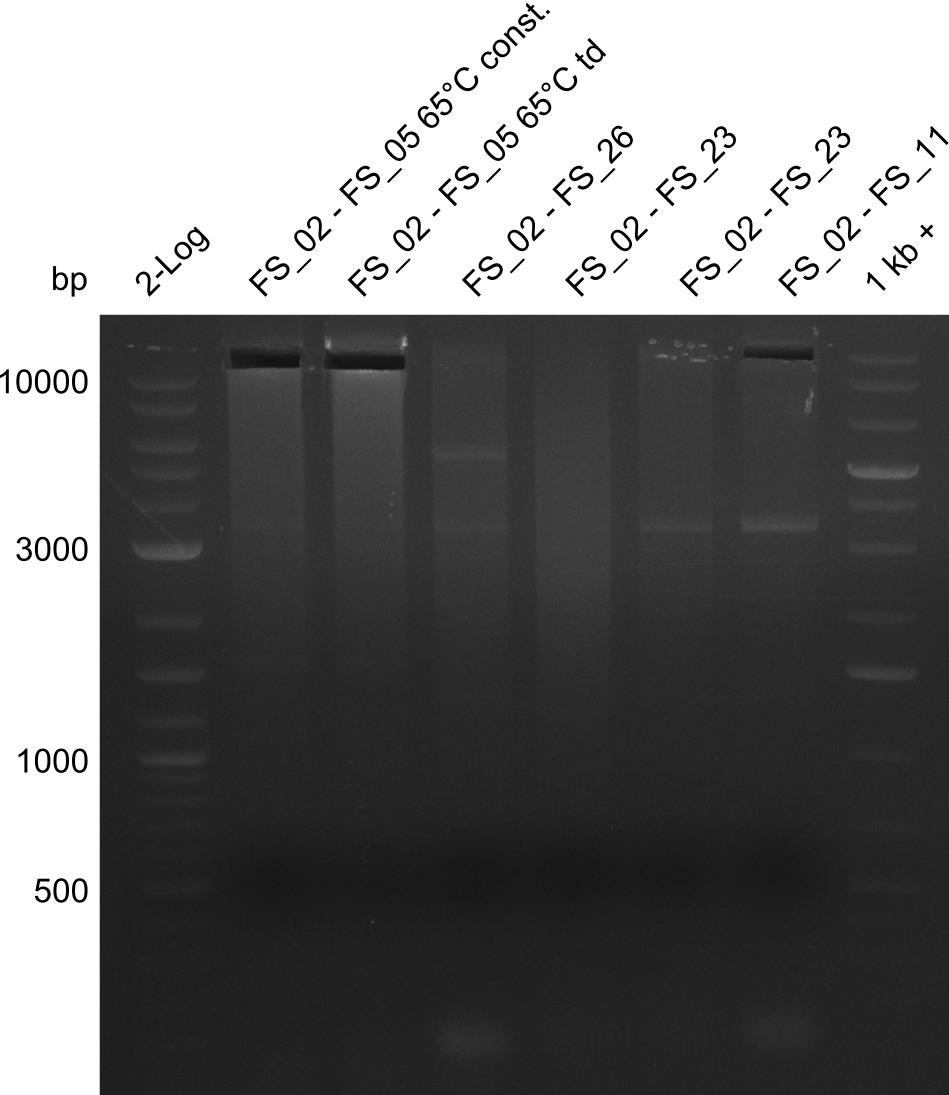
| - | [[File: | + | [[File:Heidelberg_20130811 2log AF65const AF65td FS2-26 FS02-23 FS02-23 02-11s 1kbruler.png|150px|thumb|Amplifcation of DelAF (02-05) and DelAG (2-26), (2-13), (2-11s) ; run at 135 V, 0.8 % gel (TAE)(10.08)]] |
| - | [[File: | + | [[File:Heidelberg_20130811 2log AF65const AF65td FS2-26 FS02-23 FS02-23 02-11s 1kbruler cut.png|150px|thumb|Amplifcation of DelAF (02-05) and DelAG (2-26), (2-13), (2-11s) after cutting ; run at 135 V, 0.8 % gel (TAE)(10.08)]] |
:'''Reaction''' | :'''Reaction''' | ||
| Line 326: | Line 326: | ||
===Amplification from FS_02 to FS_05; 11.2 kb=== | ===Amplification from FS_02 to FS_05; 11.2 kb=== | ||
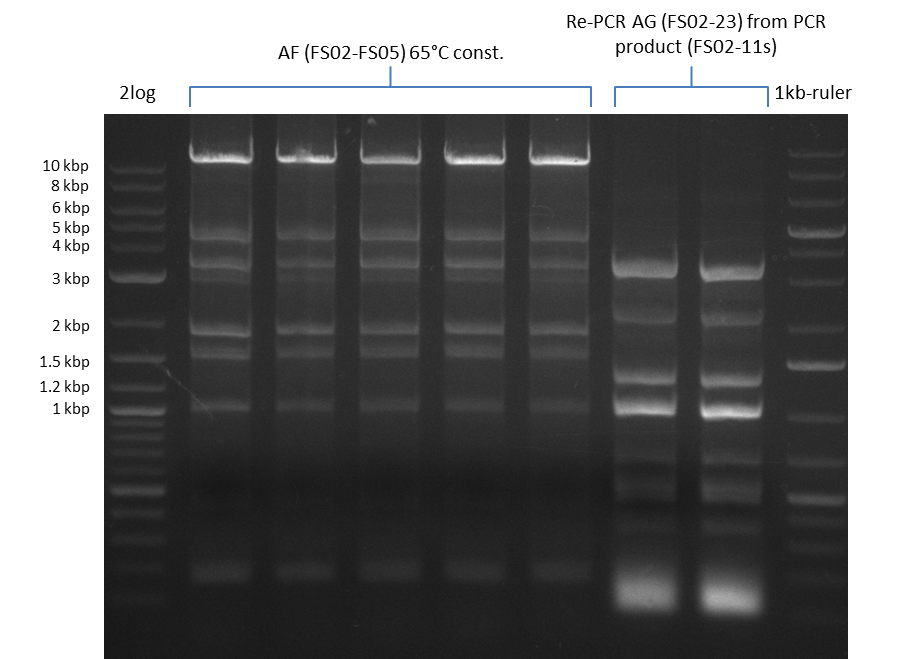
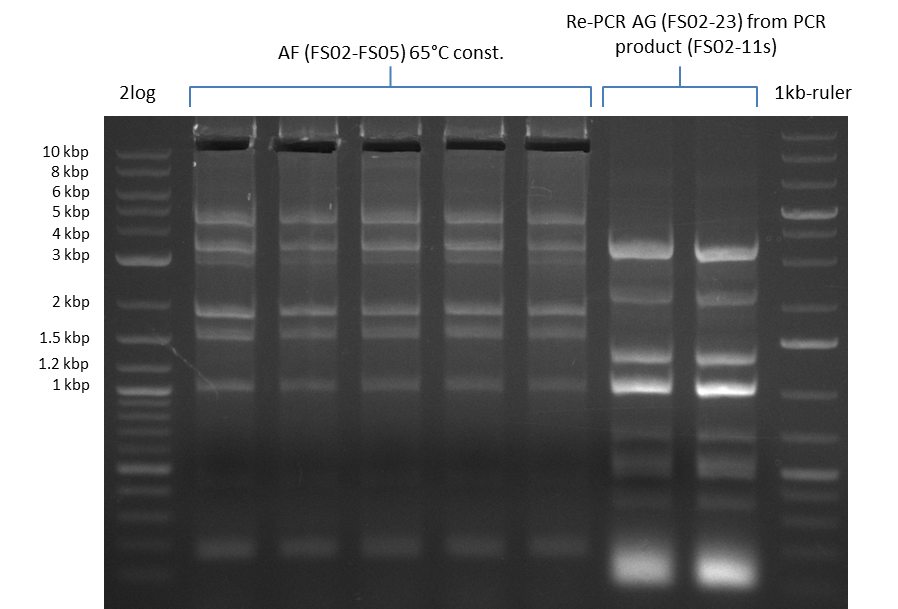
| - | [[File: | + | [[File:Heidelberg_20130812 2log 5xAF(FS02-FS05)@62°Cconst RePCRDelAG(FS02-FS23)withFS02-11sastemplate 1,10Template 1,20Template 1kbruler.png|150px|thumb| Amplification of DelAF @62°C const., Re-PCR of DelAG with 1:10 and 1:20 deluted Template from 10.08; run at 135 V, 0.8 % gel (TAE)(10.08)]] |
| - | [[File: | + | [[File:Heidelberg_20130812 2log 5xAF(FS02-FS05)@62°Cconst RePCRDelAG(FS02-FS23)withFS02-11sastemplate 1,10Template 1,20Template 1kbruler cut.png|150px|thumb| Amplification of DelAF @62°C const., Re-PCR of DelAG with 1:10 and 1:20 deluted Template from 10.08 after cutting; run at 135 V, 0.8 % gel (TAE)(10.08)]] |
Revision as of 01:47, 30 September 2013
Contents |
07-08-2013
Amplification I from FS_02 to FS_05; 11.2 kb
- Reaction
| what | µL |
|---|---|
| D. acidovorans SPH-1 | 1 |
| FS_02 (1/10) | 2.5 |
| FS_05 (1/10) | 2.5 |
| Phusion Master Mix | 25 |
| DMSO | 2.5 |
| dd H2O | 16.5 |
- Conditions
| Biometra TProfessional Basic | ||
|---|---|---|
| Cycles-PCR | temperature [°C] | Time [s] |
| 1 | 98 | 10 |
| 12 | 98 | 1 |
| 68 ↓ 0.5 | 5 | |
| 72 | 3 min | |
| 18 | 98 | 1 |
| 66 | 5 | |
| 72 | 3 min | |
| 1 | 72 | 10 min |
| 1 | 4 | inf |
Results:
- Amplification of DelAF did not work
- Repeat amplification in case any errors were made
Amplification II from FS_02 to FS_05; 11.2 kb
- Reaction
| what | µL |
|---|---|
| D. acidovorans SPH-1 | 1 |
| FS_02 (1/10) | 2.5 |
| FS_05 (1/10) | 2.5 |
| Phusion Master Mix | 25 |
| DMSO | 2.5 |
| dd H2O | 16.5 |
- Conditions
| Biorad MyCycler* | ||
|---|---|---|
| Cycles-PCR | temperature [°C] | Time [s] |
| 1 | 98 | 10 |
| 12 | 98 | 1 |
| 68 ↓ 0.5 | 5 | |
| 72 | 3 min | |
| 18 | 98 | 1 |
| 66 | 5 | |
| 72 | 3 min | |
| 1 | 72 | 10 min |
| 1 | 4 | inf |
Results:
- Amplification of DelAF did not work as no product appeared, therefore conditions of PCR have to be optimized
- as extremely long amplicons in the past were usually easier to amplify from agar plate (colony PCR) the same conditions will be tried on the colony as template
08-08-2013
Amplification from FS_02 to FS_05; 11.2 kb
- Reaction
| what | µL |
|---|---|
| D. acidovorans SPH-1 (from agarplate) | 1 |
| FS_02 (1/10) | 2.5 |
| FS_05 (1/10) | 2.5 |
| Phusion Master Mix | 25 |
| DMSO | 2.5 |
| dd H2O | 16.5 |
- Conditions
| Biorad MyCycler* | ||
|---|---|---|
| Cycles-PCR | temperature [°C] | Time [s] |
| 1 | 98 | 10 |
| 12 | 98 | 1 |
| 68 ↓ 0.5 | 5 | |
| 72 | 3 min | |
| 18 | 98 | 1 |
| 66 | 5 | |
| 72 | 3 min | |
| 1 | 72 | 10 min |
| 1 | 4 | inf |
Results:
- Amplification of DelAF did not work as no product appeared, therefore conditions of PCR have to be optimized
- PCR will be repeated with glycerol stock as template
09-08-2013
Amplification from FS_02 to FS_05; 11.2 kb
- Reaction
| what | µL 2nd PCR |
|---|---|
| D. acidovorans SPH-1 (glycerol stock) | 1 |
| FS_02 (1/10) | 2.5 |
| FS_05 (1/10) | 2.5 |
| Phusion Master Mix | 25 |
| DMSO | 2.5 |
| dd H2O | 16.5 |
- Conditions
| Biorad MyCycler | ||
|---|---|---|
| Cycles-PCR | temperature [°C] | Time [s] |
| 1 | 98 | 10 |
| 12 | 98 | 1 |
| 68 ↓ 0.5 | 5 | |
| 72 | 3 min | |
| 18 | 98 | 1 |
| 66 | 5 | |
| 72 | 3 min | |
| 1 | 72 | 10 min |
| 1 | 10 | inf |
Results:
- Amplification of DelAF did not work as no product appeared, therefore conditions of PCR have to be optimized
- PCR will be repeated with a higher annealing temperature as primers might not have bound due to secondary structes
- PCR will be repeated with lower annealing temperatue as primers might not have bound due to high temperatures
10-08-2013
Amplification from FS_02 to FS_05; 11.2 kb
- Reaction
| Reagent | DelAF | |
|---|---|---|
| Template | D.acidovorans SPH-1 colony | |
| Primer fw | 2 µL FS_02 | 4 µL FS_02 |
| Primer rev | 2 µL FS_05 | 4 µL FS_05 |
| Phusion flash Ready Mix | 10 µL | |
| dd H2O | 1 µL | - |
- Conditions
| Biorad T100 | ||
|---|---|---|
| Cycles | temperature [°C] | Time [s] |
| 1 | 98 | 10 |
| 30 | 98 | 1 |
| 58 | 5 | |
| 72 | 3:15 | |
| 1 | 72 | 10 min |
| 1 | 10 | inf |
Results:
- Amplification of DelAF did not work at a temperature of 58°C constant
- as primers might not have been bound at this low temperatue but neither bound at a temperature of 68°C (touchdown or constant), PCR will be repeated at a temperature of 65°C as touchdown and at constant annealing
Amplification II + III from FS_02 to FS_05; 11.2 kb
- Reaction
| Reagent | DelAF II + III | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Template | D.acidovorans SPH-1 colony | |||||||||
| Primer fw | 4.5 µL FS_02 | |||||||||
| Primer rev | 4.5 µL FS_05 | |||||||||
| DMSO | 1 µL | |||||||||
| Phusion Ready Mix | 10 µL | |||||||||
- Conditions
| Biorad MyCycler | ||||||
|---|---|---|---|---|---|---|
| Cycles | temperature [°C] DelAF II | Time | Cycles | temperature [°C] DelAF III | Time | |
| 1 | 98 | 10 s | 1 | 98 | 10 s | |
| 30 | 98 | 1 s | 12 | 98 | 1 s | |
| 65 ↓ 0.5 | 5 s | |||||
| 65 | 5 s | 72 | 3:15 min | |||
| 18 | 98 | 1 s | ||||
| 72 | 3:15 min | 66 | 5 s | |||
| 72 | 3:15 min | |||||
| 1 | 72 | 10 min | 1 | 72 | 10 min | |
| 1 | 10 | inf | 1 | 10 | inf | |
Results:
- Amplification of DelAF from the new strain finally worked, both conditions (65°C constant and 65°C touchdown) led to specific product but the touchdown PCR resulted in a smear
- bands were cut out and DNA purified using QIAquick Gel Extraction Kit
- PCR will be repeated with a slightly lower temperature with constant annealing to increase yield but avoid the smear which appeared due to the low annealing temperatures used in the last cycles of the touchdown PCR.
11-08-2013
Amplification from FS_02 to FS_05; 11.2 kb
- Reaction
| Reagent | DelAF |
|---|---|
| Template | D.acidovorans SPH-1 colony |
| Primer fw | 4.5 µL FS_02 |
| Primer rev | 4.5 µL FS_05 |
| Phusion Ready Mix | 10 µL |
- Conditions
| Biorad T100 | ||
|---|---|---|
| Cycles | temperature [°C] | Time [s] |
| 1 | 98 | 10 |
| 30 | 98 | 1 |
| 62 | 5 | |
| 72 | 3:15 | |
| 1 | 72 | 10 min |
| 1 | 10 | inf |
Results:
- Amplification of DelAF worked out though again a smear and several other bands appeared
- bands were cut out very precise to avoid carry over of any unwanted amplicon and DNA purified using QIAquick Gel Extraction Kit
- gradient PCR will be carried out to determine optimal annealing temperature of primers and thereby obtain product of higher specificity suitable for gibson assembly
 "
"