Team:Heidelberg/Templates/Del week15 FG
From 2013.igem.org
(Difference between revisions)
(→Restriction digest of fragment from FS_21 to FS_26; 5.5 kb; 29-07-2013) with ClaI) |
(→Concentration measurement DelFG) |
||
| Line 32: | Line 32: | ||
! Fragment !! Primer !! Date PCR !! Concentration | ! Fragment !! Primer !! Date PCR !! Concentration | ||
|- | |- | ||
| - | | DelFG || FS21-FS26 | | + | | DelFG || FS21-FS26 || 07-08-2013 || 24.3 ng/µl |
|} | |} | ||
Latest revision as of 19:04, 3 October 2013
Contents |
08-08-2013
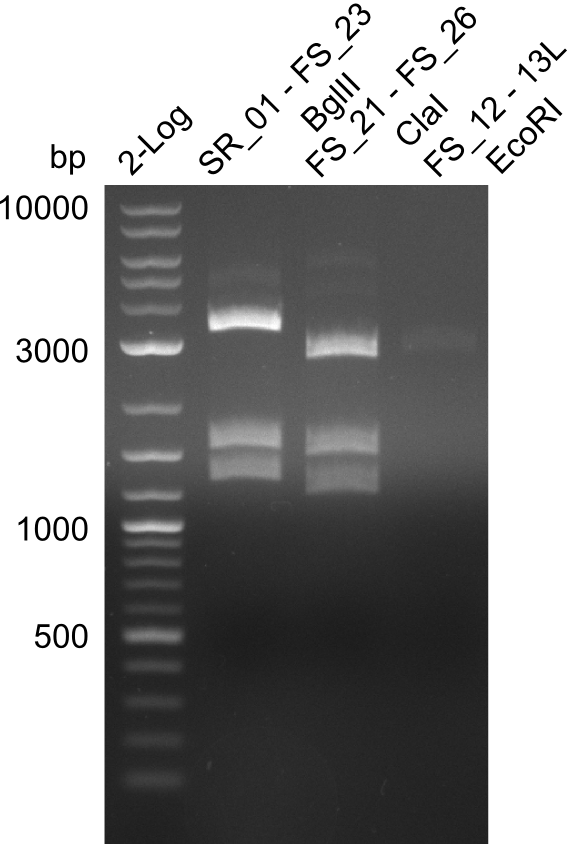
Restriction digest of fragment from FS_21 to FS_26; 5.5 kb; 29-07-2013) with ClaI
Incubation at Room temperature for about 4h and at 37°C for 1 hours
| what | µl |
|---|---|
| FS_21 to FS_26 (29-07-2013) | 10 |
| ClaI | 1 |
| Buffer CutSmart | 1.5 |
| dd H2O | 2.5 |
| Expected fragment lengths [bp] | 2743, 1519, 1208 |
Results:
- restriction digest of DelFG led to the expected fragment sizes, therefore restriction digest, implies amplification of the correct fragment
- PCR product will be further validated by single read sequencing before Gibson Assembly
Concentration measurement DelFG
| Fragment | Primer | Date PCR | Concentration |
|---|---|---|---|
| DelFG | FS21-FS26 | 07-08-2013 | 24.3 ng/µl |
09-08-2013
Amplification from FS_21 to FS_26; 5.5 kb
- Reaction
| what | µl |
|---|---|
| D. acidovorans SPH-1 (colony-pcr) | 1 |
| FS_21: (1/10) | 2 |
| FS_26: (1/10) | 2 |
| Phusion flash Master Mix | 10 |
| DMSO | 1 |
| dd H2O | 4 |
- Conditions I
| Biorad T100 | ||
|---|---|---|
| Cycles | temperature [°C] | Time [s] |
| 1 | 98 | 10 |
| 30 | 98 | 1 |
| 60 | 5 | |
| 72 | 2:15 | |
| 1 | 72 | 10 min |
| 1 | 10 | inf |
Results:
- Amplification of DelFG was successful
- bands were cut out and DNA purified using QIAquick Gel Extraction Kit
 "
"