Team:Heidelberg/Templates/Del week16 AF
From 2013.igem.org
(Difference between revisions)
(Created page with "==12-08-2013== ===Amplification from FS_02 to FS_05; 11.2 kb=== [[File:20130812 2log 5xAF(FS02-FS05)gradient 67.5 67.0 66.5 66.0 65.5 3xOP(FS22-13L)gradientfromPCR-product(FS22-...") |
|||
| Line 2: | Line 2: | ||
===Amplification from FS_02 to FS_05; 11.2 kb=== | ===Amplification from FS_02 to FS_05; 11.2 kb=== | ||
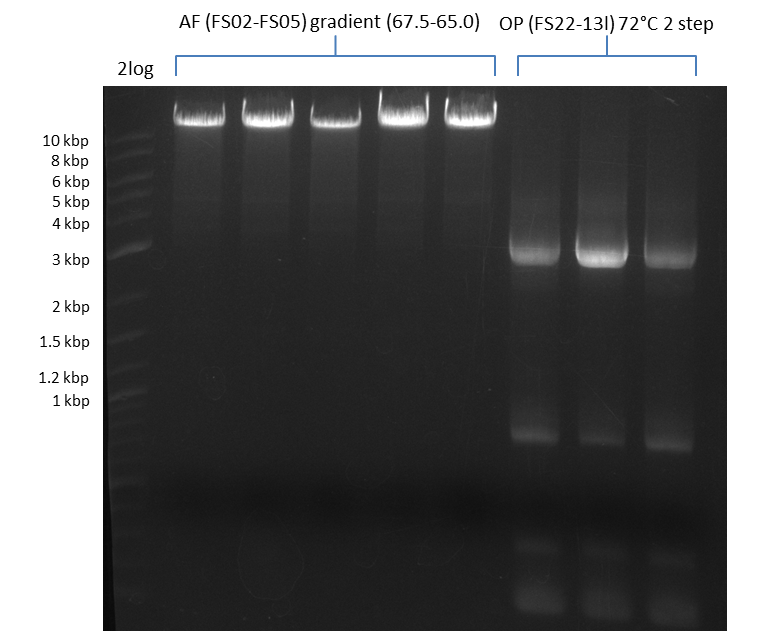
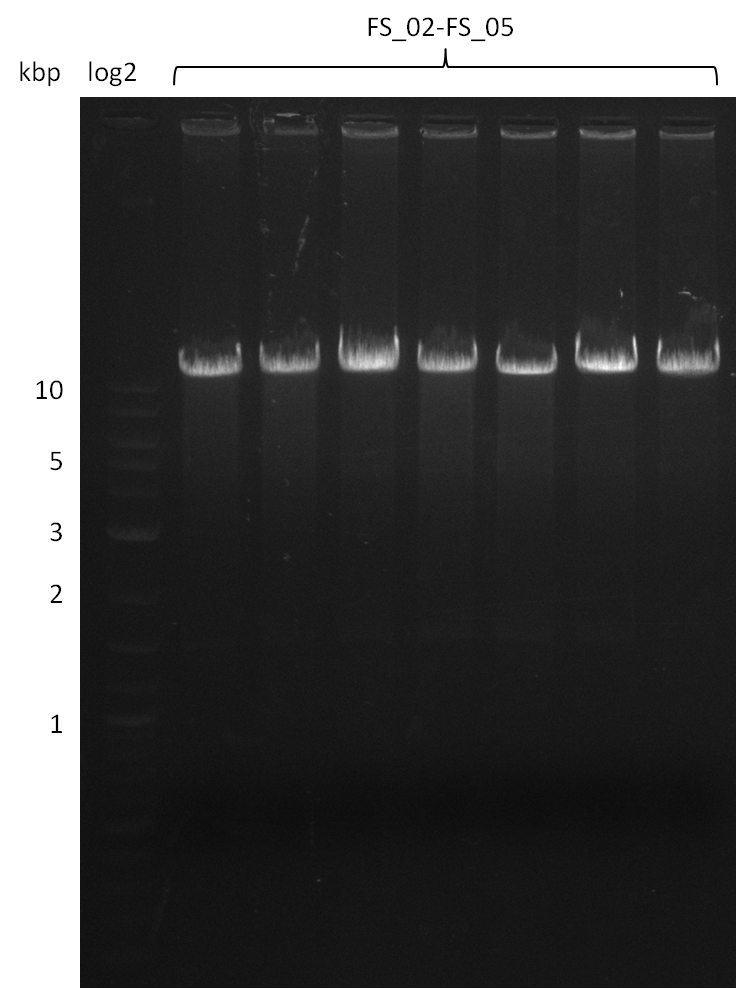
| - | [[File: | + | [[File:Heidelberg_20130812 2log 5xAF(FS02-FS05)gradient 67.5 67.0 66.5 66.0 65.5 3xOP(FS22-13L)gradientfromPCR-product(FS22-13s)72°C2step.png|150px|thumb| Amplification of DelAF using gradient PCR, Amplification of DelOP 72°C 2-step; run at 135 V, 0.8 % gel (TAE)(11.08)]] |
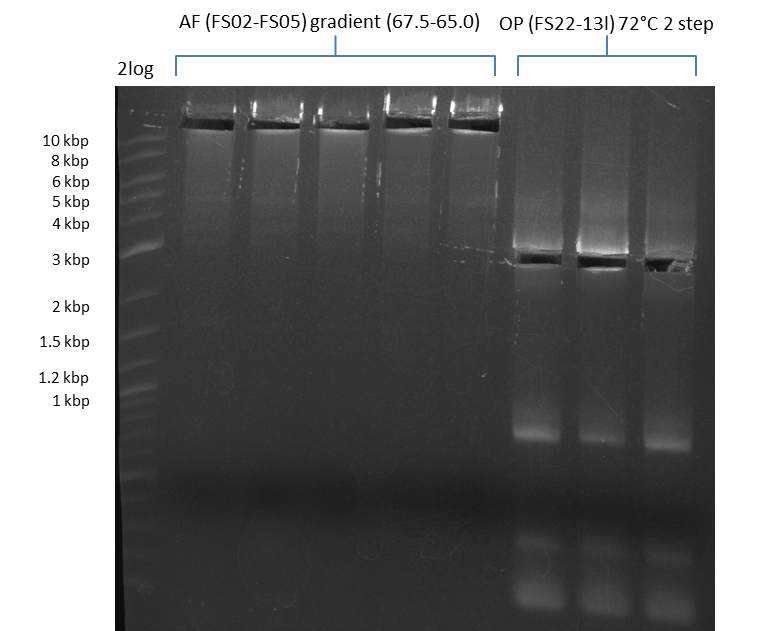
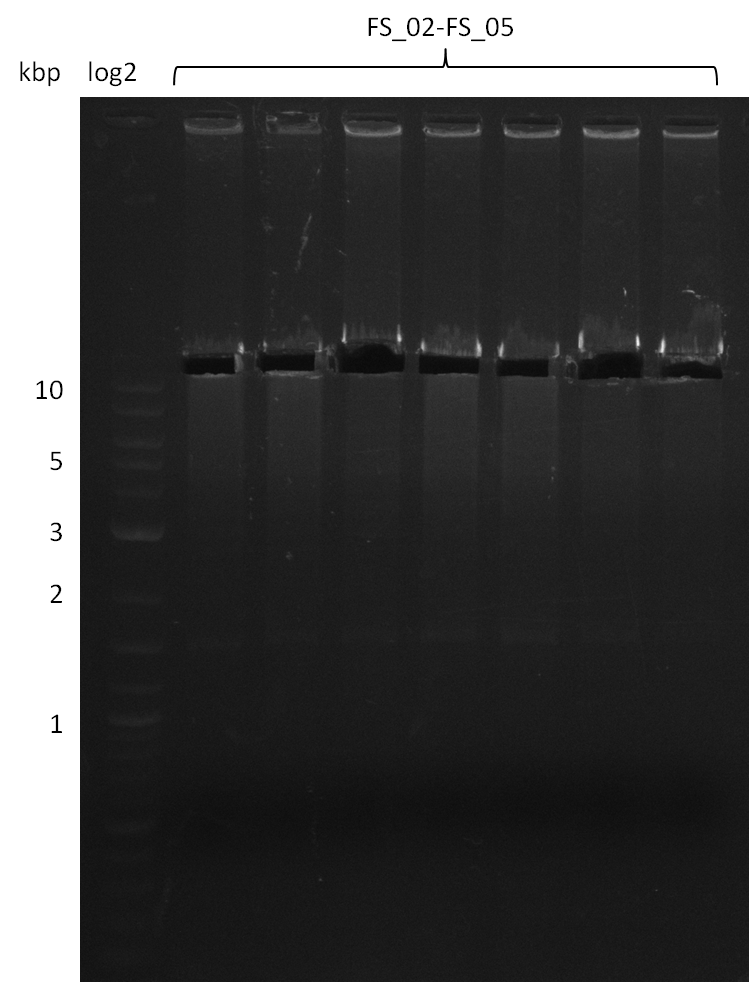
| - | [[File: | + | [[File:Heidelberg_20130812 2log 5xAF(FS02-FS05)gradient 67.5 67.0 66.5 66.0 65.5 3xOP(FS22-13L)gradientfromPCR-product(FS22-13s)72°C2step cut.png|150px|thumb| Amplification of DelAF using gradient PCR, Amplification of DelOP 72°C 2-step after cutting; run at 135 V, 0.8 % gel (TAE)(11.08)]] |
:'''Reaction''' | :'''Reaction''' | ||
| Line 82: | Line 82: | ||
===Amplification from FS_02 to FS_05; 11.2 kb=== | ===Amplification from FS_02 to FS_05; 11.2 kb=== | ||
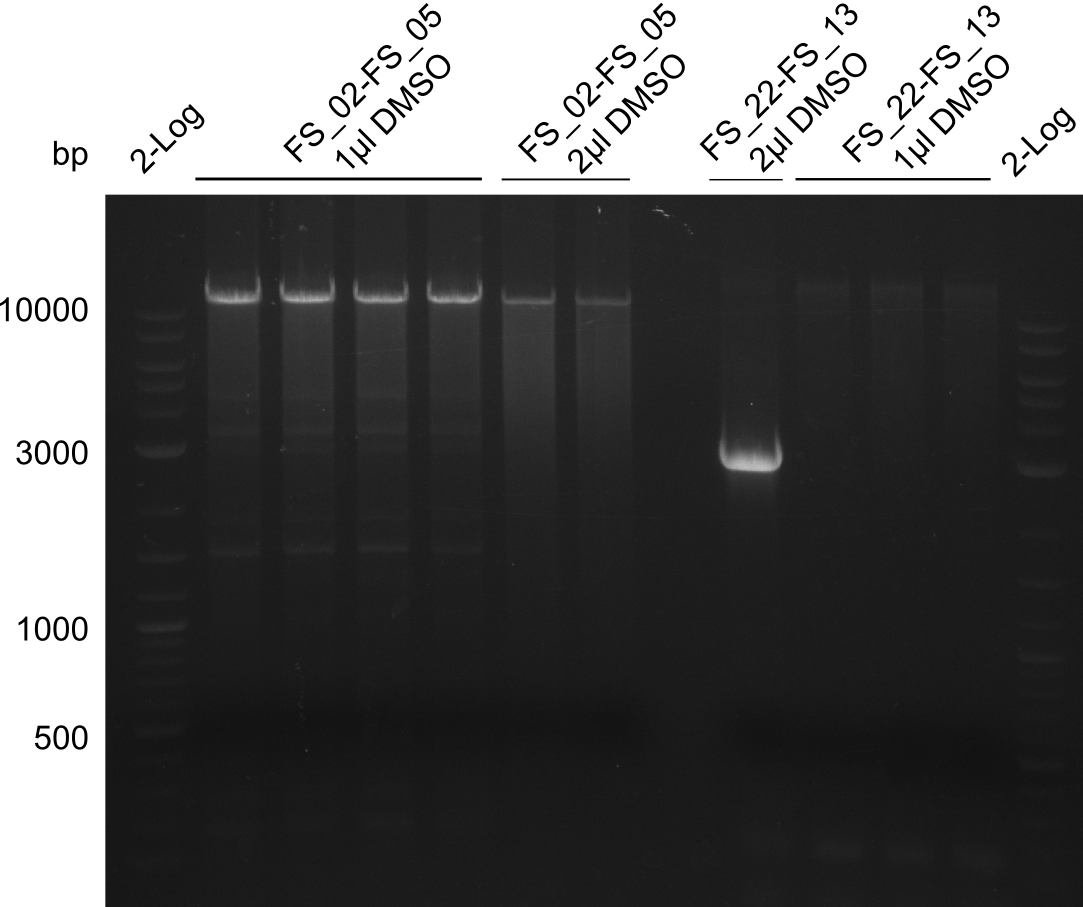
| - | [[File: | + | [[File:Heidelberg_20130814 2log 4xAF(FS02-05) AF(Fs02-05)highDMSO AF(FS02-05)MM empty OP2µLDMSO 3xOP1µLDMSO 2log.png|150px|thumb|Amplification of DelAF (FS_02-FS_05); run at 100 V, 0.8 % gel (TAE)(13.08)]] |
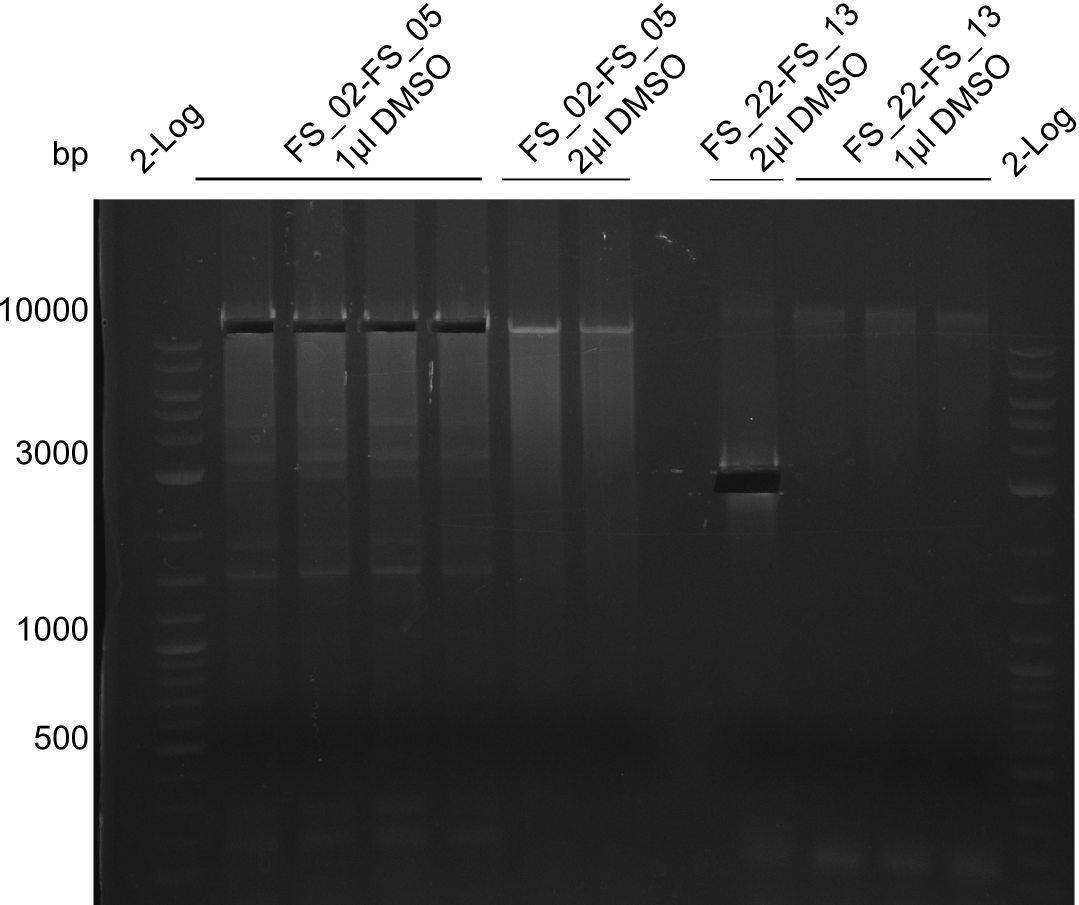
| - | [[File: | + | [[File:Heidelberg_20130814 2log 4xAF(FS02-05) AF(Fs02-05)highDMSO AF(FS02-05)MM empty OP2µLDMSO 3xOP1µLDMSO 2log cut.png|150px|thumb|Amplification of DelAF (FS_02-FS_05), cut; run at 100 V, 0.8 % gel (TAE)(13.08)]] |
:'''Reaction''' | :'''Reaction''' | ||
| Line 154: | Line 154: | ||
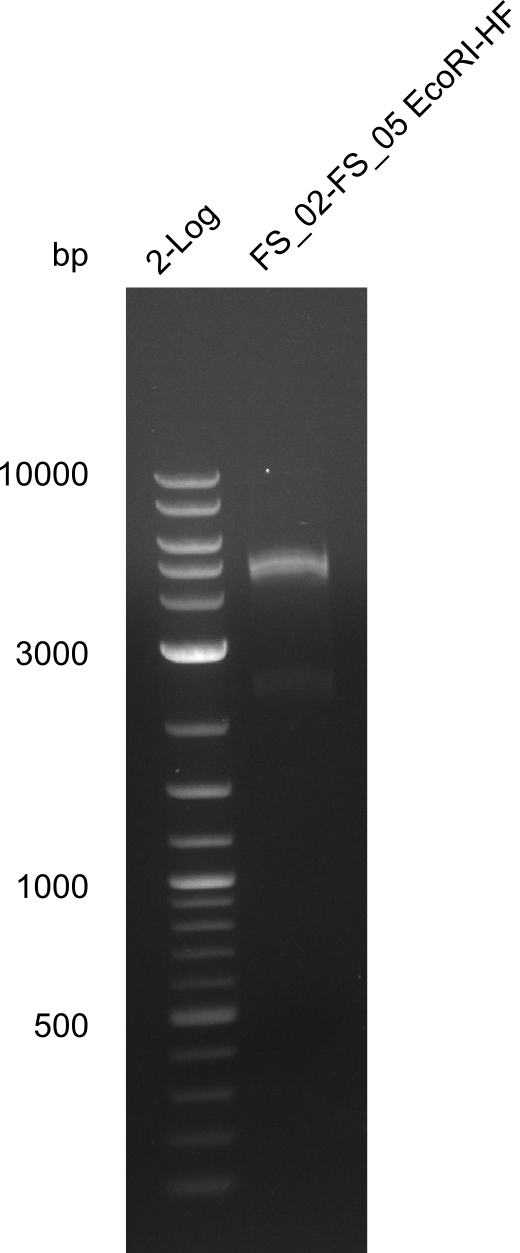
===Restriction digest of fragment FS_02 to FS_05; 11.2 kb; [[DelA-F#11-08-2013|11-08-2013]] with EcoRI-HF=== | ===Restriction digest of fragment FS_02 to FS_05; 11.2 kb; [[DelA-F#11-08-2013|11-08-2013]] with EcoRI-HF=== | ||
| - | [[File: | + | [[File:Heidelberg_20130814 log2 DigestAFEcoRI.png|150px|thumb|Test restriction digest of fragment FS_02-FS_05 (12-08) with EcoRI; run at 100 V, 0.8 % gel (TAE)(13.08)]] |
| Line 180: | Line 180: | ||
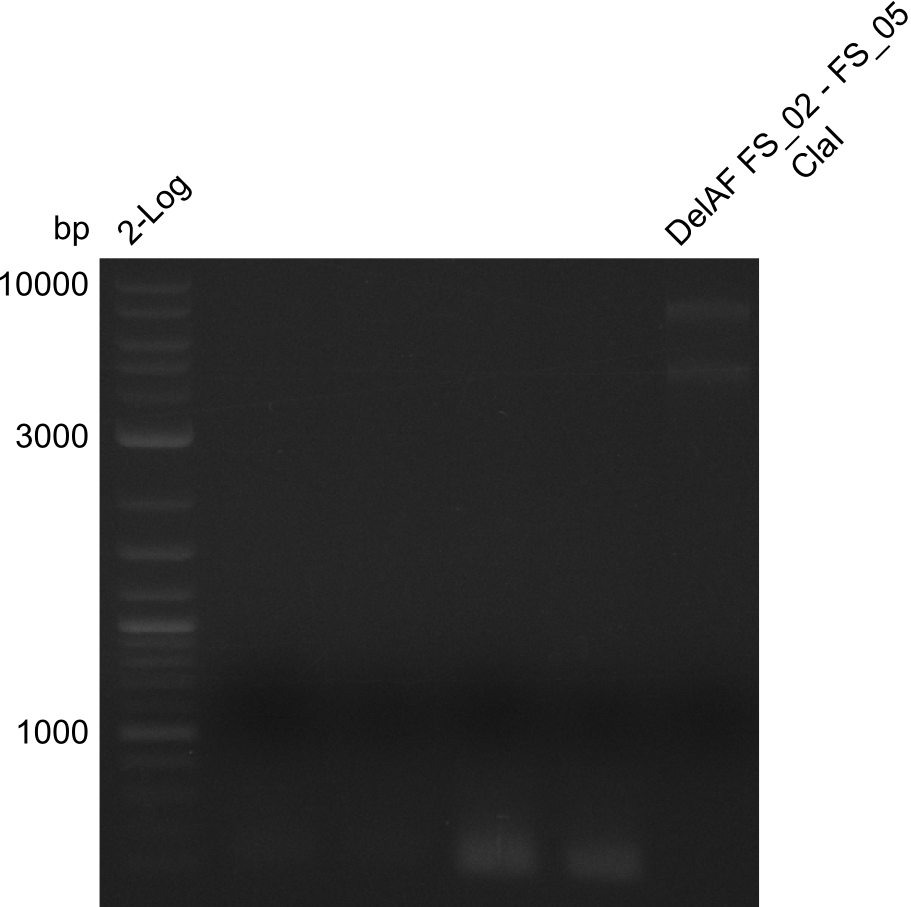
===Restriction digest of fragment FS_02 to FS_05; 11.2 kb; [[DelA-F#10-08-2013|10-08-2013]] with ClaI=== | ===Restriction digest of fragment FS_02 to FS_05; 11.2 kb; [[DelA-F#10-08-2013|10-08-2013]] with ClaI=== | ||
| - | [[File: | + | [[File:Heidelberg_20130814 DelAF digest ClaI.png|150px|thumb|Test restriction digest of fragment FS_02-FS_05 (12-08) with ClaI; run at 100 V, 0.8 % gel (TAE)(14.08)]] |
Incubation at 37°C for 2 hours | Incubation at 37°C for 2 hours | ||
| Line 205: | Line 205: | ||
==17-08-2013== | ==17-08-2013== | ||
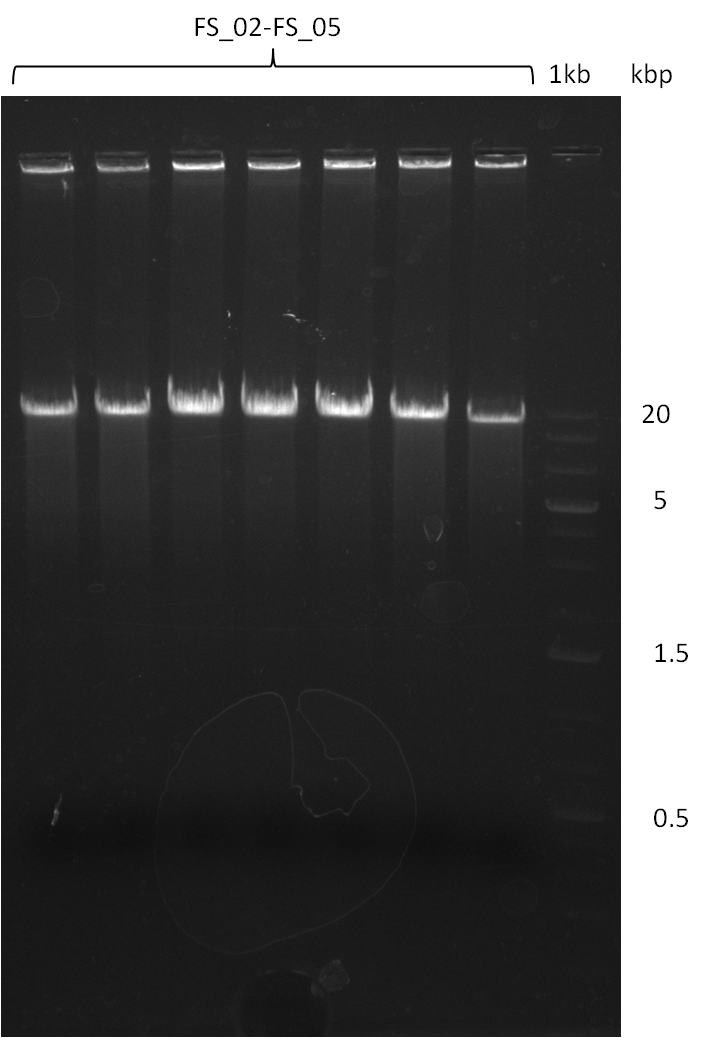
===Amplification from FS_02 to FS_05; 11.2 kb=== | ===Amplification from FS_02 to FS_05; 11.2 kb=== | ||
| - | [[File: | + | [[File:Heidelberg_20130817 7xAF 1kbpbesch.png|150px|thumb| Amplification of DelAF (17-08); run at 100 V, 0.8 % gel (TAE)]] |
| - | [[File: | + | [[File:Heidelberg_20130817 7xAF 1kbpcut.png|150px|thumb| Amplification of DelAF, cut (17-08); run at 100 V, 0.8 % gel (TAE)]] |
:'''Reaction of DelAF''' | :'''Reaction of DelAF''' | ||
| Line 261: | Line 261: | ||
==18-08-2013== | ==18-08-2013== | ||
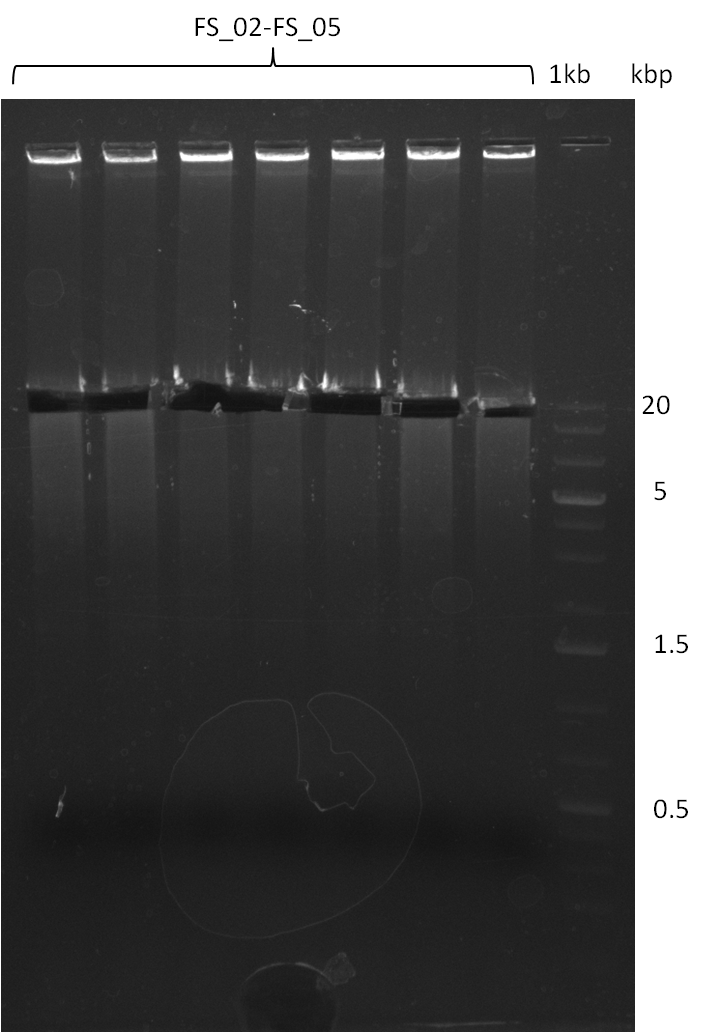
===Amplification from FS_02 to FS_05; 11.2 kb=== | ===Amplification from FS_02 to FS_05; 11.2 kb=== | ||
| - | [[File: | + | [[File:Heidelberg_20130818 2log 7xDelAFbesch.png|150px|thumb| Amplification of DelAF (18-08); run at 100 V, 0.8 % gel (TAE)]] |
| - | [[File: | + | [[File:Heidelberg_20130818 2log 7xDelAF cutbesch.png|150px|thumb| Amplification of DelAF, cut (18-08); run at 100 V, 0.8 % gel (TAE)]] |
:'''Reaction of DelAF''' | :'''Reaction of DelAF''' | ||
Revision as of 01:49, 30 September 2013
Contents |
12-08-2013
Amplification from FS_02 to FS_05; 11.2 kb
- Reaction
| Reagent | DelAF | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Template | D.acidovorans SPH-1 colony | |||||||||
| Primer fw | 4.5 µL FS_02 | |||||||||
| Primer rev | 4.5 µL FS_05 | |||||||||
| DMSO | 1 µL | |||||||||
| Phusion Ready Mix | 10 µL | |||||||||
- Conditions
| Biometra TProfessional Basic | ||
|---|---|---|
| Cycles | temperature [°C] | Time [s] |
| 1 | 98 | 10 |
| 12 | 98 | 1 |
| 67.5 - 65.0 (ΔT = 0.5) ↓ 0.5 | 5 | |
| 72 | 3:20 | |
| 18 | 98 | 1 |
| 65.5 - 63.0 (ΔT = 0.5) | 5 | |
| 72 | 3:20 | |
| 1 | 72 | 10min |
| 1 | 10 | inf |
Results:
- Amplification of DelAF worked well, gradient displays an optimal annealing temperature of 65.5°C, which will be used for further amplifications
- bands were cut out and DNA purified using QIAquick Gel Extraction Kit
13-08-2013
Restriction digest of fragment FS_02 to FS_05; 11.2 kb; 12-08-2013 with EcoRI-HF
Incubation at 37°C for 2 hours
| what | µL |
|---|---|
| FS_02 to FS_05 (12-08-2013) | 20 |
| EcoRI-HF | 1 |
| CutSmart Buffer | 2.5 |
| dd H2O | 1.5 |
Expected fragment sizes: 2.26kbp; 4.62kbp; 4.32kbp
Results:
- Weak bands of about 4.6kbp visible, as well as on of about 2.6kbp
- digest will be repeated using higher concentrations of DNA to clearify results of restriction digest
Amplification from FS_02 to FS_05; 11.2 kb
- Reaction
1 sample contains 2µL of DMSO
| Reagent | DelAF | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Template | D.acidovorans SPH-1 colony | |||||||||
| Primer fw | 4.5 µL FS_02 | |||||||||
| Primer rev | 4.5 µL FS_05 | |||||||||
| DMSO | 1 µL | |||||||||
| Phusion Ready Mix | 10 µL | |||||||||
- Conditions
| Biorad T100 | ||
|---|---|---|
| Cycles | temperature [°C] | Time [s] |
| 1 | 98 | 10 |
| 12 | 98 | 1 |
| 65.5 ↓ 0.5 | 5 | |
| 72 | 3:20 | |
| 18 | 98 | 1 |
| 63.5 | 5 | |
| 72 | 3:20 | |
| 1 | 72 | 10min |
| 1 | 10 | inf |
Results:
- Amplification of DelAF worked though a slight smear occured, therefore PCR will be repeated on the more precise Biometra TProfessional Basic cylcer again
- bands were cut out and DNA purified using QIAquick Gel Extraction Kit
14-08-2013
Concentration measurement (FS_02 to FS_05; 11.2 kb)
| Fragment | Primer | Date PCR | Concentration |
|---|---|---|---|
| DelAF | FS02-FS05 | 11-08-2013 | ~10 ng/µL |
| DelAF | FS02-FS05 | 12-08-2013 | 0 ng/µL |
| DelAF | FS02-FS05 | 12-08-2013 | 0 ng/µL |
Results:
- concentrations are not sufficient for gibson assembly
- PCR will be repeated and gel slices of different reactions will be pooled for one gel extraction using QIAquick Gel Extraction Kit to obtain the concentrations needed for gibson assembly
Restriction digest of fragment FS_02 to FS_05; 11.2 kb; 11-08-2013 with EcoRI-HF
Incubation at 37°C for 2 hours 45min
| what | µL |
|---|---|
| FS_02 to FS_05 (11-08-2013) | 18 |
| EcoRI-HF | 1 |
| CutSmart Buffer | 2.5 |
| dd H2O | 3.5 |
Expected fragment sizes: 2.26kbp; 4.62kbp; 4.32kbp
Results:
- One band of about 5kbp and one of 2.5kbp
- no clear result, digest will be repeated with another enzyme (ClaI), as the enzyme used was beyond expiration date
Restriction digest of fragment FS_02 to FS_05; 11.2 kb; 10-08-2013 with ClaI
Incubation at 37°C for 2 hours
| what | µL |
|---|---|
| FS_02 to FS_05 (10-08-2013) | 20 |
| ClaI | 1 |
| CutSmart Buffer | 2.5 |
| dd H2O | 1.5 |
Expected fragment sizes: 6.9kbp, 4.3kbp
Results:
- restriction digest displays the expected fragments, therefore amplification of the desired fragment can be assumed
- PCR product will be prepared for sequencing by GATC to proof amplification of the desired DNA sequence before Gibson Assembly
17-08-2013
Amplification from FS_02 to FS_05; 11.2 kb
- Reaction of DelAF
6x20 µL
| Reagent | DelAF | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Template | D.acidovorans SPH-1 colony | |||||||||
| Primer fw | 2.5 µL FS_02 | |||||||||
| Primer rev | 2.5 µL FS_05 | |||||||||
| Phusion Ready Mix | 10 µL | |||||||||
| DMSO | 1 µL | |||||||||
| dd H2O | 4 µL | |||||||||
- Conditions
| Biometra TProfessional Basic | ||
|---|---|---|
| Cycles | temperature [°C] | Time [s] |
| 1 | 98 | 10 |
| 12 | 98 | 1 |
| 65.5 ↓ 0.5 | 5 | |
| 72 | 3:20 | |
| 18 | 98 | 1 |
| 63.5 | 5 | |
| 72 | 3:20 | |
| 1 | 72 | 10min |
| 1 | 10 | inf |
Results:
- Amplification of DelAF worked
- bands were cut out, pooled and DNA purified using QIAquick Gel Extraction Kit to obtain concentrations needed for gibson assembly
18-08-2013
Amplification from FS_02 to FS_05; 11.2 kb
- Reaction of DelAF
6x20 µL
| Reagent | DelAF | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Template | D.acidovorans SPH-1 colony | |||||||||
| Primer fw | 2.5 µL FS_02 | |||||||||
| Primer rev | 2.5 µL FS_05 | |||||||||
| DMSO | 1 µL | |||||||||
| Phusion Ready Mix | 10 µL | |||||||||
| dd H2O | 4 µL | |||||||||
- Conditions
| Biometra TProfessional Basic | ||
|---|---|---|
| Cycles | temperature [°C] | Time [s] |
| 1 | 98 | 10 |
| 12 | 98 | 1 |
| 65.5 ↓ 0.5 | 5 | |
| 72 | 3:20 | |
| 18 | 98 | 1 |
| 63.5 | 5 | |
| 72 | 3:20 | |
| 1 | 72 | 10min |
| 1 | 10 | inf |
Results:
- Amplification of DelAF worked
- bands were cut out and DNA purified using QIAquick Gel Extraction Kit
 "
"