Team:Heidelberg/Templates/MM week18p
From 2013.igem.org
(Difference between revisions)
Nils.kurzawa (Talk | contribs) m (Created page with " == 2013-08-26 == [[File:Methylmalonyl-digest2013-08-26.png|150px|thumb|left|Lane 1: NEB 2-Log, Lane 2: pIK6.1 digested with HindIII+BamHI; lane 3: pIK6.2 digested with HindIII+B...") |
|||
| Line 1: | Line 1: | ||
| - | |||
== 2013-08-26 == | == 2013-08-26 == | ||
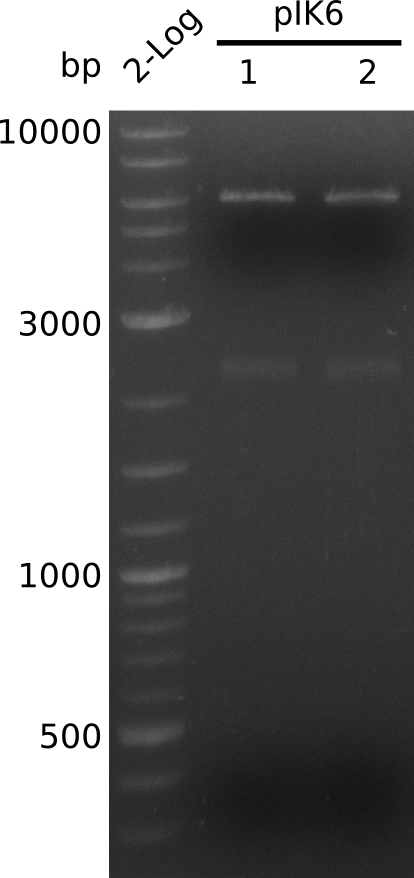
| - | [[File: | + | [[File:Heidelberg_Methylmalonyl-digest2013-08-26.png|150px|thumb|left|Lane 1: NEB 2-Log, Lane 2: pIK6.1 digested with HindIII+BamHI; lane 3: pIK6.2 digested with HindIII+BamHI]] |
* make miniPreps of colonies 1 and 2: 71.4 ng/µl and 75.1 ng/µl in 38 µl | * make miniPreps of colonies 1 and 2: 71.4 ng/µl and 75.1 ng/µl in 38 µl | ||
* digest with HindIII+BamHI (3 µl DNA, 0.5 µl of each enzyme, 20 µl total); expected: 4.1 kb, 2.1 kb, 0.9 kb | * digest with HindIII+BamHI (3 µl DNA, 0.5 µl of each enzyme, 20 µl total); expected: 4.1 kb, 2.1 kb, 0.9 kb | ||
* no 0.9 kb fragment => no BamHI site in KanR gene, 2.1 fragment visible corresponds to acca-sfp => insert completely within backbone | * no 0.9 kb fragment => no BamHI site in KanR gene, 2.1 fragment visible corresponds to acca-sfp => insert completely within backbone | ||
* co-transform TOP10 with pIK6.1+pRB21 | * co-transform TOP10 with pIK6.1+pRB21 | ||
| - | * sequences of pIK1.3 arrived: [[:File: | + | * sequences of pIK1.3 arrived: [[:File:Heidelberg_PIK1.3-2013-08-26.zip|PIK1.3-2013-08-26.zip]], [[:File:Heidelberg_PIK1.3-2013-08-26 VF2.clustal.txt|PIK1.3-2013-08-26 VF2.clustal.txt]], [[:File:Heidelberg_PIK1.3-2013-08-26 VR.clustal.txt|PIK1.3-2013-08-26 VR.clustal.txt]] |
* point mutation in permeability device, interestingly, both the frameshift in pIK1.2 and this point mutation create a stop codon at the beginning of the permeability device | * point mutation in permeability device, interestingly, both the frameshift in pIK1.2 and this point mutation create a stop codon at the beginning of the permeability device | ||
* send pIK1.4 to sequencing with primer VF2 | * send pIK1.4 to sequencing with primer VF2 | ||
== 2013-08-27 == | == 2013-08-27 == | ||
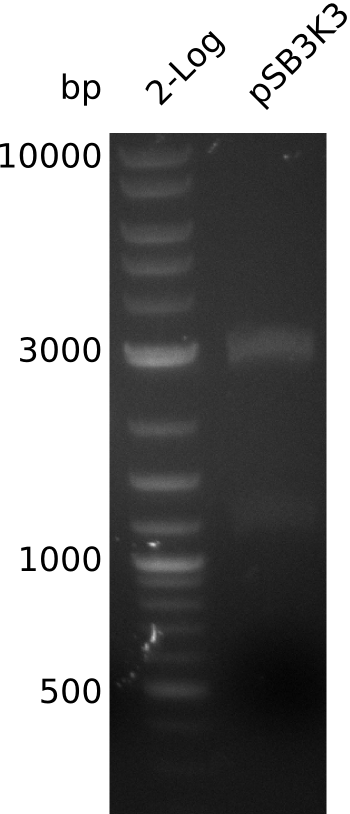
| - | [[File: | + | [[File:Heidelberg_Methylmalonyl-digest2013-08-28.png|150px|thumb|right|Lane 1: NEB 2-log, lane 2: pSB3K3-BBa_J04450 digested with EcoRI+SpeI]] |
* TOP10-pIK6.1-pRB21 are not blue, but the indigoidine group is having problems with pRB21 -> pRB21 might not work | * TOP10-pIK6.1-pRB21 are not blue, but the indigoidine group is having problems with pRB21 -> pRB21 might not work | ||
| - | * sequence of pIK1.4 arrived: [[:File: | + | * sequence of pIK1.4 arrived: [[:File:Heidelberg_PIK1.4-2013-08-27.zip|PIK1.4-2013-08-27.zip]], [[:File:Heidelberg_PIK1.4-2013-08-27 VF2.clustal.txt|PIK1.4-2013-08-27 VF2.clustal.txt]] |
* again frame-shift in permeability device -> send pIK1.6, pIK1.7, pIK1.10 to sequencing with primer VF2 | * again frame-shift in permeability device -> send pIK1.6, pIK1.7, pIK1.10 to sequencing with primer VF2 | ||
* make miniPrep of pSB3K3: 54.7 ng/µl in 38 µl | * make miniPrep of pSB3K3: 54.7 ng/µl in 38 µl | ||
| Line 34: | Line 33: | ||
== 2013-08-28 == | == 2013-08-28 == | ||
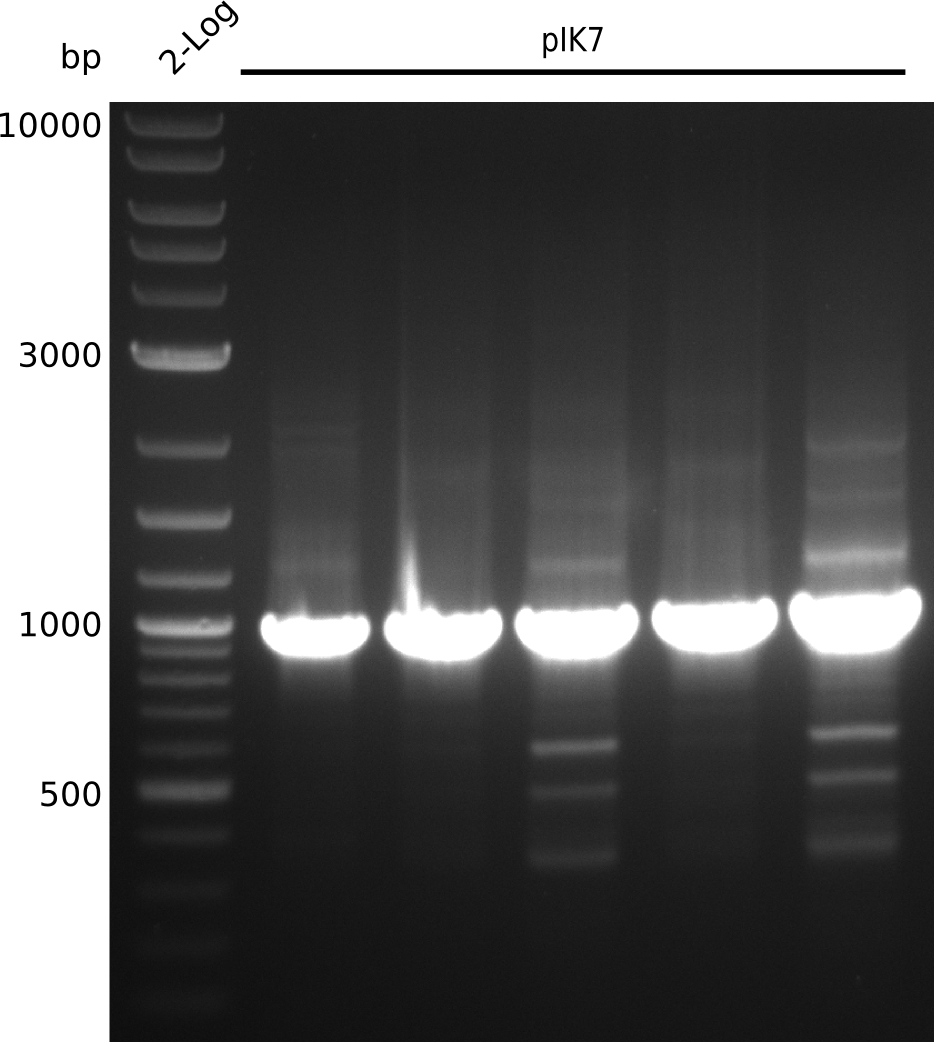
| - | [[File: | + | [[File:Heidelberg_Methylmalonyl-colony-PCR2013-08-28.png|150px|thumb|left|Colony-PCR of TOP10 transformed with pSB3K3 ligated with pIK2 (-> pIK7) and primers VF2+IK25. Lane 1: NEB 2-log; lanes 2-6: pIK7]] |
* pick colonies, run colony-PCR with primers VF2+IK25 (expected: 1kb, iTaq, 20 µl total volume): | * pick colonies, run colony-PCR with primers VF2+IK25 (expected: 1kb, iTaq, 20 µl total volume): | ||
{| class="wikitable" | {| class="wikitable" | ||
| Line 55: | Line 54: | ||
== 2013-08-29 == | == 2013-08-29 == | ||
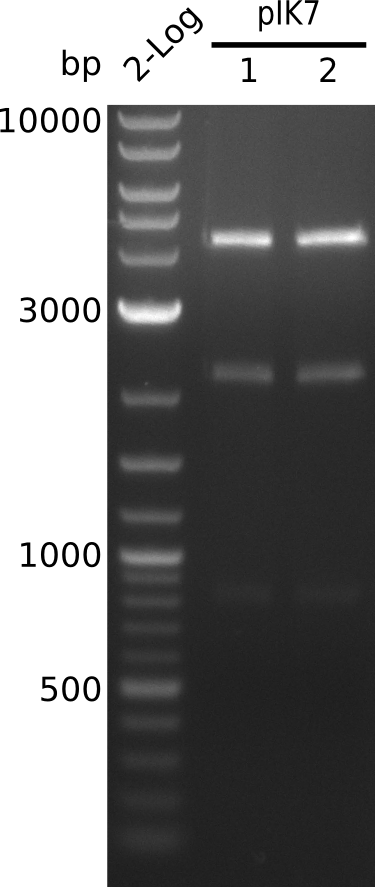
| - | [[File: | + | [[File:Heidelberg_Methylmalonyl-digest2013-08-29.png|150px|thumb|left|Lane 1: NEB 2-Log, Lane 2: pIK7.1 digested with HindIII+BamHI; lane 3: pIK7.2 digested with HindIII+BamHI]] |
| - | * sequences of pIK1 arrived: [[:File: | + | * sequences of pIK1 arrived: [[:File:Heidelberg_PIK1.6-pIK1.7-pIK1.10-2013-08-29.zip|PIK1.6-pIK1.7-pIK1.10-2013-08-29.zip]], [[:File:Heidelberg_PIK1.6-2013-08-29 VF2.clustal.txt|PIK1.6-2013-08-29 VF2.clustal.txt]], [[:File:Heidelberg_PIK1.7-2013-08-29 VF2.clustal.txt|PIK1.7-2013-08-29 VF2.clustal.txt]], [[:File:Heidelberg_PIK1.10-2013-08-29 VF2.clustal.txt|PIK1.10-2013-08-29 VF2.clustal.txt]] |
* frame-shift in pIK1.6, point mutations in pIK1.7, pIK1.10 | * frame-shift in pIK1.6, point mutations in pIK1.7, pIK1.10 | ||
* perform miniPreps of pIK7 -> ca. 50 ng/µl in 38 µl | * perform miniPreps of pIK7 -> ca. 50 ng/µl in 38 µl | ||
| Line 77: | Line 76: | ||
* expected fragments: 2151 bp + 7265 bp | * expected fragments: 2151 bp + 7265 bp | ||
<gallery widths=150px> | <gallery widths=150px> | ||
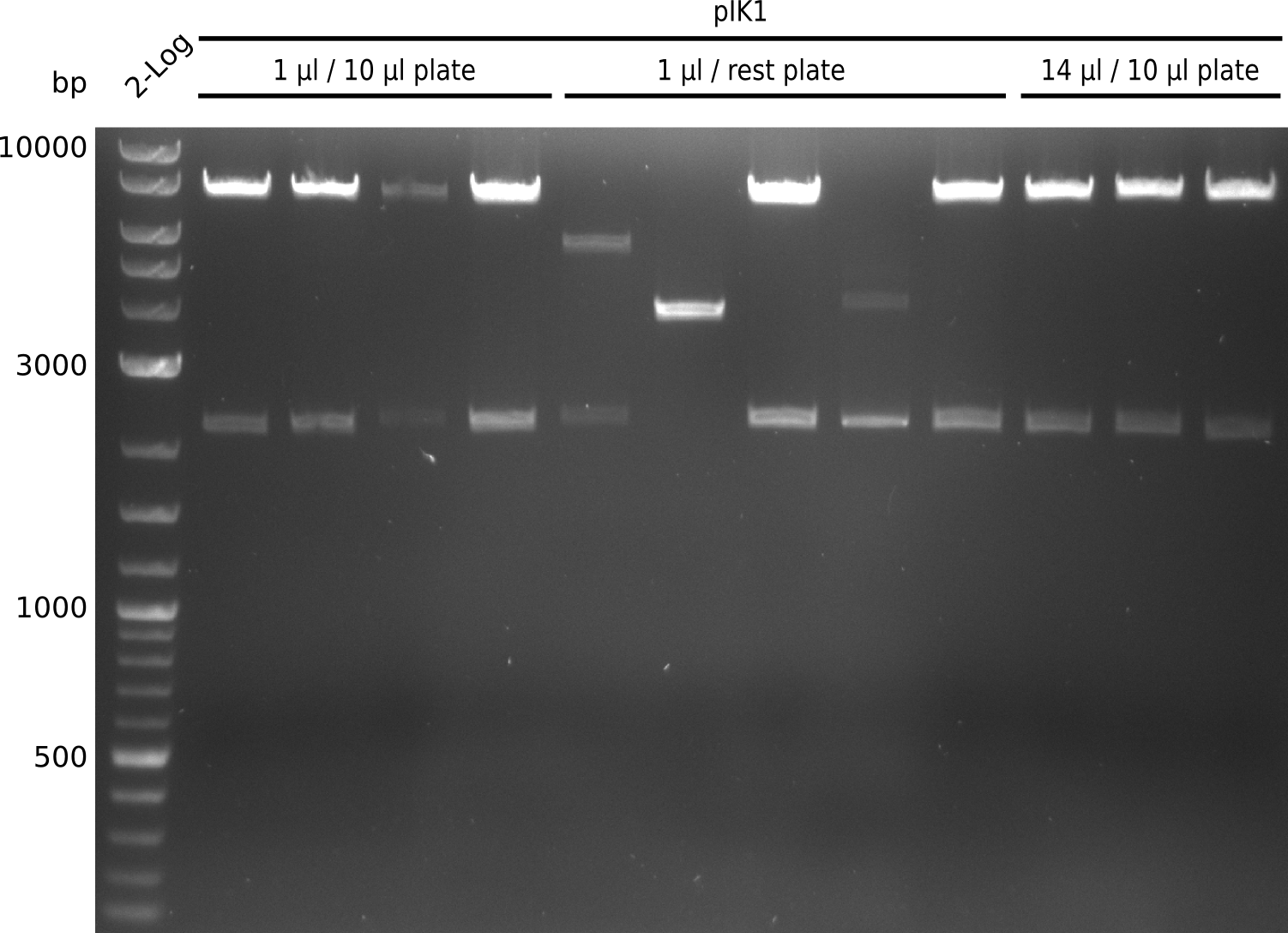
| - | File: | + | File:Heidelberg_Methylmalonyl-digest2013-09-01_1.png|Lane 1: NEB 2-log; lanes 2-13: pIK1 miniPreps digested with BamHI+HindIII; lanes 2-5: 1 µl / 10 µl plate; lanes 6-10: 1 µl / rest plate; lanes 11-13: 14 µl / 10 µl plate |
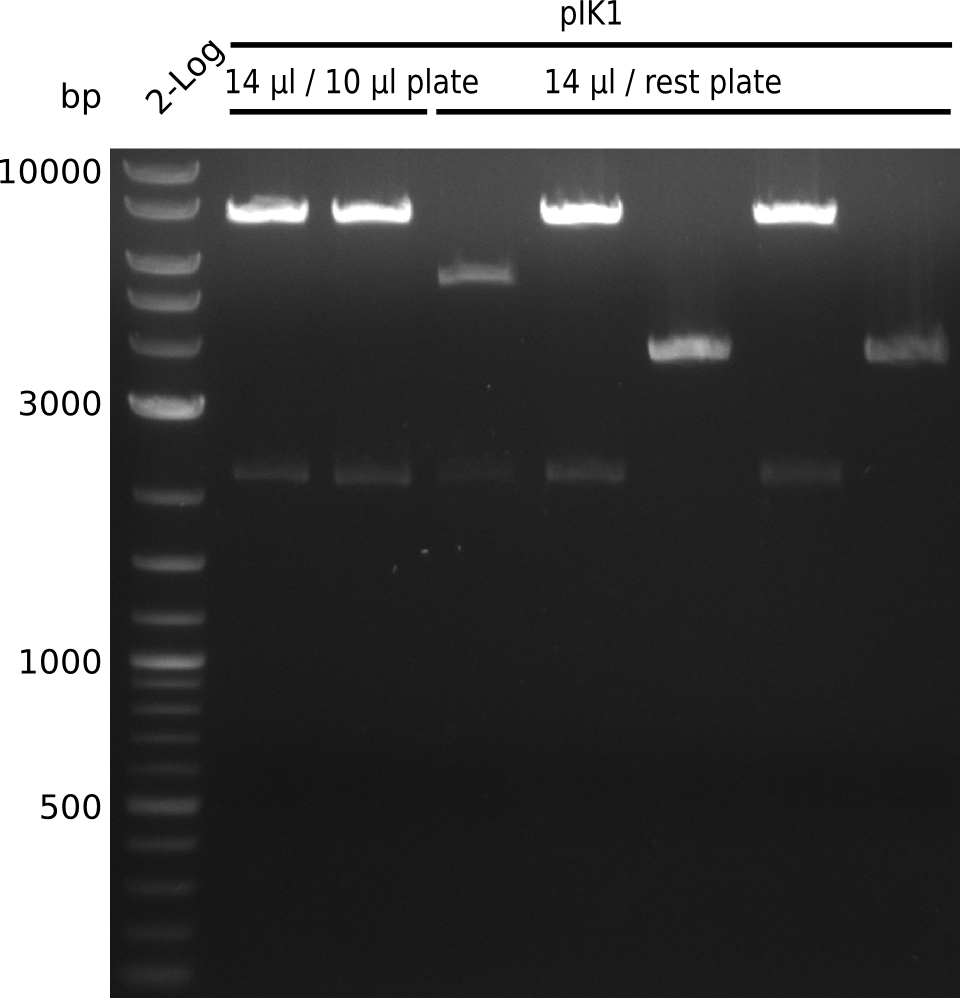
| - | File: | + | File:Heidelberg_Methylmalonyl-digest2013-09-01_2.png|Lane 1: NEB 2-log; lanes 2-8: pIK1 miniPreps digested with BamHI+HindIII; lanes 2-3: 14 µl / 10 µl plate; lanes 4-8: 14 µl / rest plate |
</gallery> | </gallery> | ||
Revision as of 23:36, 4 October 2013
Contents |
2013-08-26
- make miniPreps of colonies 1 and 2: 71.4 ng/µl and 75.1 ng/µl in 38 µl
- digest with HindIII+BamHI (3 µl DNA, 0.5 µl of each enzyme, 20 µl total); expected: 4.1 kb, 2.1 kb, 0.9 kb
- no 0.9 kb fragment => no BamHI site in KanR gene, 2.1 fragment visible corresponds to acca-sfp => insert completely within backbone
- co-transform TOP10 with pIK6.1+pRB21
- sequences of pIK1.3 arrived: PIK1.3-2013-08-26.zip, PIK1.3-2013-08-26 VF2.clustal.txt, PIK1.3-2013-08-26 VR.clustal.txt
- point mutation in permeability device, interestingly, both the frameshift in pIK1.2 and this point mutation create a stop codon at the beginning of the permeability device
- send pIK1.4 to sequencing with primer VF2
2013-08-27
- TOP10-pIK6.1-pRB21 are not blue, but the indigoidine group is having problems with pRB21 -> pRB21 might not work
- sequence of pIK1.4 arrived: PIK1.4-2013-08-27.zip, PIK1.4-2013-08-27 VF2.clustal.txt
- again frame-shift in permeability device -> send pIK1.6, pIK1.7, pIK1.10 to sequencing with primer VF2
- make miniPrep of pSB3K3: 54.7 ng/µl in 38 µl
- digest pSB3K3 with EcoRI+SpeI (20 µl total volume, 5 µl DNA, 0.5 µl of each enzyme)
- gel-purify, ligate with pIK2.6 fragment from 2013-08-24 at RT for 1h -> pIK7:
| what | µl |
|---|---|
| pSB3K3 | 9 |
| pIK2.6 | 8 |
| T4 ligase | 1 µl |
| T4 ligase buffer | 2 µl |
- heat-inactivate: 75°C for 5 min
- transform 10 µl of ligation into TOP10, plate on Kan, grow at 37°C
2013-08-28
- pick colonies, run colony-PCR with primers VF2+IK25 (expected: 1kb, iTaq, 20 µl total volume):
| Cycles | temperature [°C] | Time [s] |
|---|---|---|
| 1 | 95 | 300 |
| 35 | 95 | 30 |
| 54 | 30 | |
| 72 | 90 | |
| 1 | 72 | 600 |
| 1 | 10 | inf |
- grow in 2xYT+Kan at 37°C
2013-08-29
- sequences of pIK1 arrived: PIK1.6-pIK1.7-pIK1.10-2013-08-29.zip, PIK1.6-2013-08-29 VF2.clustal.txt, PIK1.7-2013-08-29 VF2.clustal.txt, PIK1.10-2013-08-29 VF2.clustal.txt
- frame-shift in pIK1.6, point mutations in pIK1.7, pIK1.10
- perform miniPreps of pIK7 -> ca. 50 ng/µl in 38 µl
- digest 5 µl of pIK7.1, pIK7.2 with BamHI+HindIII (0.5 µl of each enzyme, 20 µl total volume)
- bands at right positions (expected: 4.1, 2.1, 0.8 kb)
- prepare glycerol stock of TOP10-pIK7.1
2013-08-30
- electroporate electrocompetent DH10ß with 1 µl, 14 µl of 1:4 Gibson mix for pIK1 from 2013-08-07
- plate 10 µl, rest of each electroporation on Cm, grow at 37°C
2013-08-31
- very large and small colonies present, after heat-schock transformation last time only large colonies present
- pick 5 small colonies from each plate, grow in 2xYT+Cm (no 2xYT left => last 3 colonies from 14 µl / rest plate grown in LB+Cm) at 37°C
2013-09-01
- pIK1.12 did not grow
- make miniPreps of all cultures -> concentrations about 200-300 ng/µl in 37.5 µl (except pIK1.14: 50 ng/µl)
- digest with BamHI+HindIII (20 µl total volume, 0.5 µl enzyme, 1 µl DNA)
- expected fragments: 2151 bp + 7265 bp
 "
"