From 2013.igem.org
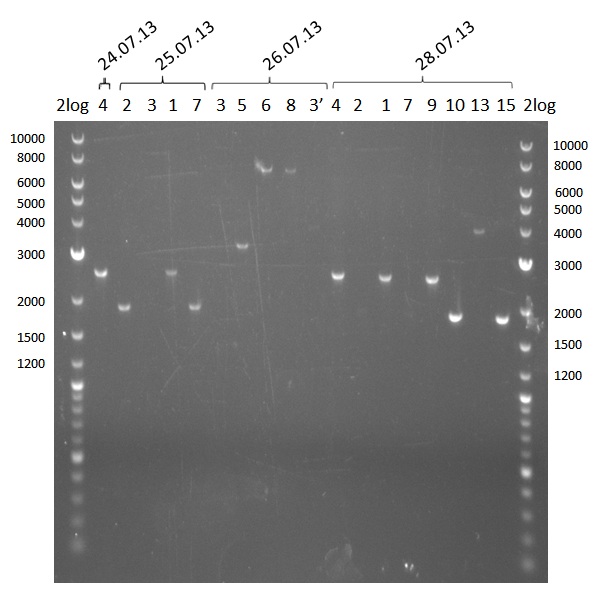
Amplifications Round I
Analysis of DNA concentrations of fragments amplified so far
Analytical Gel Electrophoresis 0.8% agarose
Scheme: each 1µL DNA (eluation in 20µL water)+ 3 µL loading dye
2log ladder, 4 µL 24.7:4, 25.7:1,2,3,7, 26.7.:3,5,6,8,3', 28.7.1,2,4,9,10,13,14 2log ladder, 4 µL
quantification gel of the fragments extracted from gel. See table for precise amounts
| fragment | concentration [ng/µl]
|
| 1 | 32
|
| 2 | 16
|
| 3 | --
|
| 4 | 30
|
| 5 | 12
|
| 6 | 8
|
| 7 | 12
|
| 8 | 8
|
| 9 | 30
|
| 10 | 40
|
| 11 | in progress
|
| 12 | in progress
|
| 13 | 4
|
| 14 | 40
|
| 15 | --
|
--> next steps:
- reamplify fragments 3, 13 & 15 (optimize)
- reamplify fragments 5, 6, 7 & 8 for higher yield
- Plan B: Re-PCR from low-yield fragments
Reamplification of low yield fragments: 2, 7, 6, 8, 5
- fragments 2 & 7 (Two-Block A)
- fragments 6 & 8 (T 100)
- fragment 5 (Two-Block B)
For all amplifications 2x 20µl PCRs were used. PCR was run with Q5
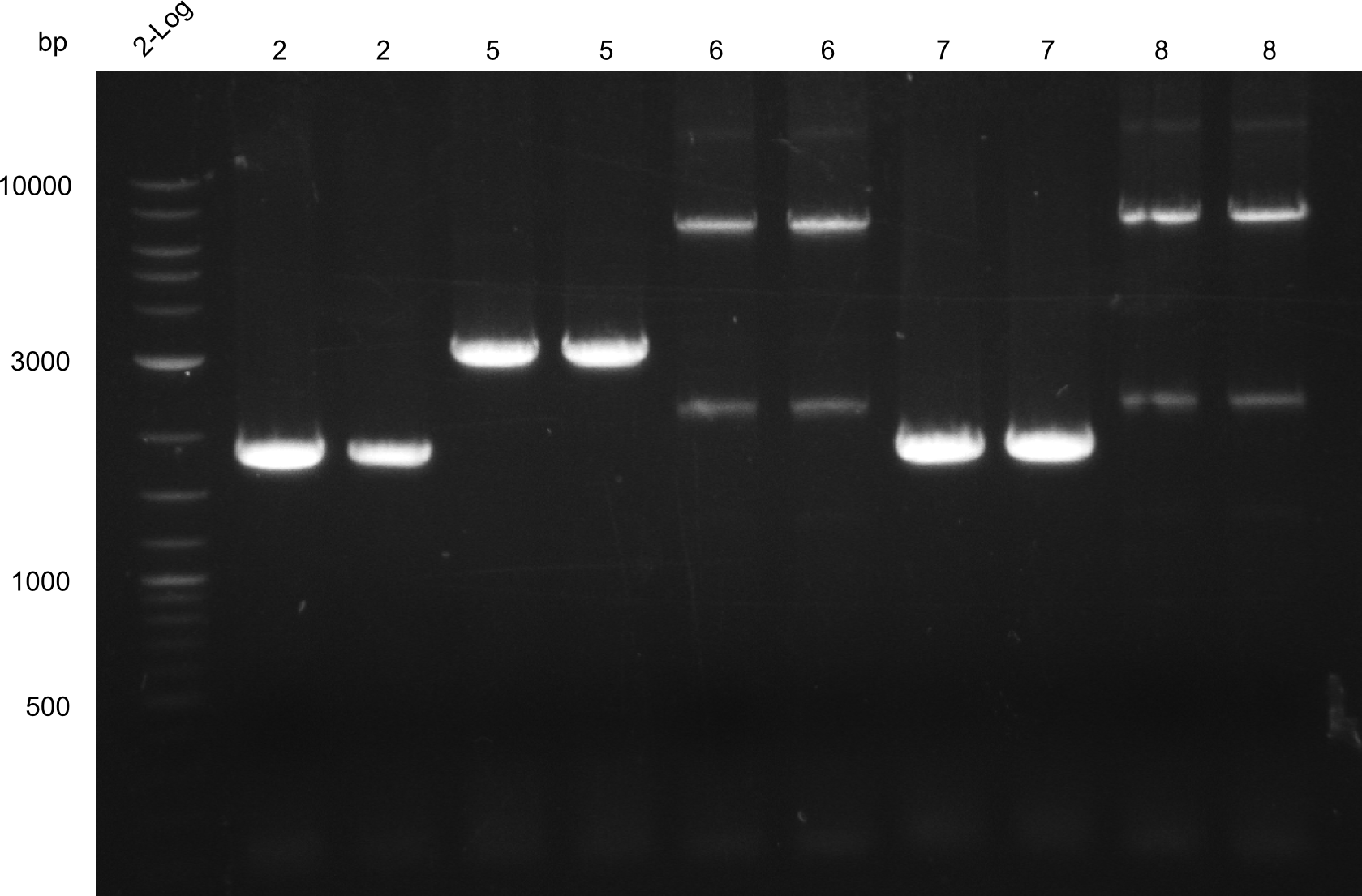
PCRs were conducted 2°C lower than Tm for all constructs. Good yields were obtained for fragments 2, 5 & 7, unspecific bands for fragments 6 & 8. All bands with right size cut out for gel-extraction.
| what | µl
|
| fw primer | 2
|
| rv primer | 2
|
| Bacillus | 1
|
| Q5 2x Master mix | 10
|
| ddH20 | 5
|
| Cycles | temperature [°C] | Time [min:s]
|
| 1 | 98 | 2:00
|
| 35 | 98 | 0:05
|
| 68 (fr. 2 & 7) / 64 (fr. 6 & 8) / 63 (fr. 5) | 0:15
|
| 72 | 1:00 (fr. 2 & 7) / 3:30 (fr. 6 & 8) / 1:30 (fr. 5)
|
| 1 | 72 | 10:00
|
| 1 | 12 | inf
|
The PCRs were run 2°C under Tm for the primers, as specific products were obtained in the previous amplifications, but yield after gel-extraction was low.
Amplification of 3, 5, 15
PCRs of Fragments 3, 5 and 15 with phusion flash. And same PCR touchdown program. Two-block A.
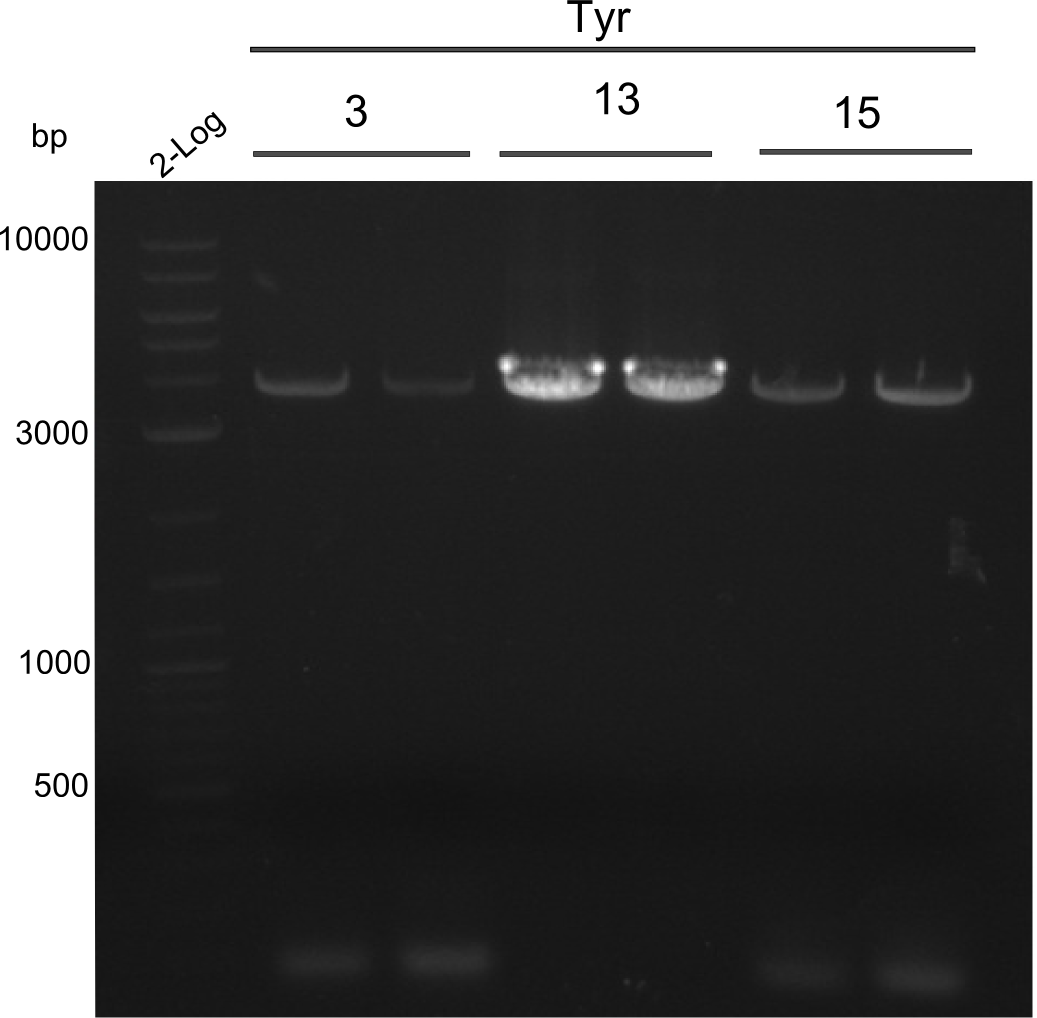
PCRs of fragments 3, 13 and 15. 2x20µl were used for increase of yield
fragment 3: IK15+13
fragment 5: IK12+PW11
fragments 15: Ik12+Pw13
| what | µl
|
| fw primer | 2
|
| rv primer | 2
|
| Bacillus | 1
|
| Phusion 2x Master mix | 10
|
| DMSO | 1
|
| ddH20 | 4
|
| Cycles | temperature [°C] | Time [s]
|
| 1 | 98 | 2:00
|
| 12 | 98 | 0:05
|
| 66↓0.5°C | 0:05
|
| 72 | 2:30
|
| 23 | 98 | 0:05
|
| 60 | 0:05
|
| 72 | 2:30
|
| 1 | 72 | 10:00
|
| 1 | 12 | inf
|
Amplification of Fragment 12
Fusion/Q5
| Cycles | temperature [°C] | Time [min:s]
|
| 1 | 98 | 2:00
|
| 36 | 98 | 0:05
|
| 67 | 0:10
|
| 72 | 1:30 (Q5) / 1:00 Fusion
|
| 1 | 72 | 10:00
|
| 1 | 12 | inf
|
Fusion Touch Down
| Cycles | temperature [°C] | Time [min:s]
|
| 1 | 98 | 2:00
|
| 12 | 98 | 2:00
|
| 68↓0.5°C | 0:05
|
| 72 | 1:00
|
| 23 | 98 | 0:05
|
| 62 | 0:05
|
| 72 | 1:00
|
| 1 | 72 | 10:00
|
| 1 | 12 | inf
|
Analysis of DNA concentrations
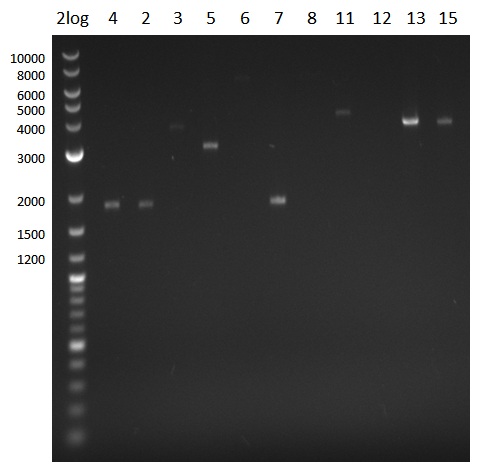
quantification gel with 4 µl 2log ladder. 1 µl sample was mixed with 3 µl loading dye
- Gel-extraction of fragments 2, 3, 4, 5, 6, 7, 8, 11, 12, 13, 15
- DNA was eluted with 30 µl instead of 20 µl
| fragment | concentration [ng/µl]
|
| 2 | 12
|
| 3 | 3
|
| 4 | 16
|
| 5 | 14
|
| 6 | --
|
| 7 | 18
|
| 8 | --
|
| 11 | 5
|
| 12 | --
|
| 13 | 20
|
| 15 | 11
|
- Reamplify fragments 6 & 8 and use gel-extraction kit for long fragments
- Optimize conditions for fragment 12
Amplification of fragments 6, 8, 12
Q5
6 IK12,18
8 IK18,20
| what | µl
|
| fw primer | 2
|
| rv primer | 2
|
| Bacillus | 1
|
| Q5 2x Master mix | 10
|
| ddH20 | 5
|
| Cycles | temperature [°C] | Time [min:s]
|
| 1 | 98 | 2:00
|
| 35 | 98 | 0:05
|
| 66 | 0:15
|
| 72 | 3:00
|
| 1 | 72 | 10:00
|
| 1 | 12 | inf
|
Phusion Flash, Touchdown
12 PW09,10
| what | µl
|
| fw primer | 2
|
| rv primer | 2
|
| Bacillus | 1
|
| Phusion 2x Master mix | 10
|
| DMSO | 1
|
| ddH20 | 4
|
| Cycles | temperature [°C] | Time [s]
|
| 1 | 98 | 2:00
|
| 12 | 98 | 2:00
|
| 66↓0.5°C | 0:05
|
| 72 | 1:10
|
| 23 | 98 | 0:05
|
| 64 | 0:05
|
| 72 | 1:10
|
| 1 | 72 | 10:00
|
| 1 | 12 | inf
|
Assembly Round I
Tripeptide-I-NRPS Assembly
Gibson Assembly to Tripeptide-I-NRPS
- DO ALL WORK ON ICE!!
- mix 1,08 µl of fragment 4, 3; 24 µl of fragment 5 & 5.68 µl of fragment 6
- add 10 µl of Gibson master mix
- let incubate for 60 minutes at 50°C
- split up 20 µl into:
- 15 µl for isopropanol / ethanol DNA precipitation
- add 60 µl (4 volumes) of isopropanol and centrifuge for 20 mins at full speed
- discard supernatant
- wash pellet in ethanol, centrifuge for 5 mins and discard supernatant, let dry
- let resuspend in 10 µl H20 and electroporate
- to the remaining 5 µl, add 10µl ddH2O
- electroporate with 1 µl
- electroporate with 14 µl
- use a negative control (10 µl H2O)
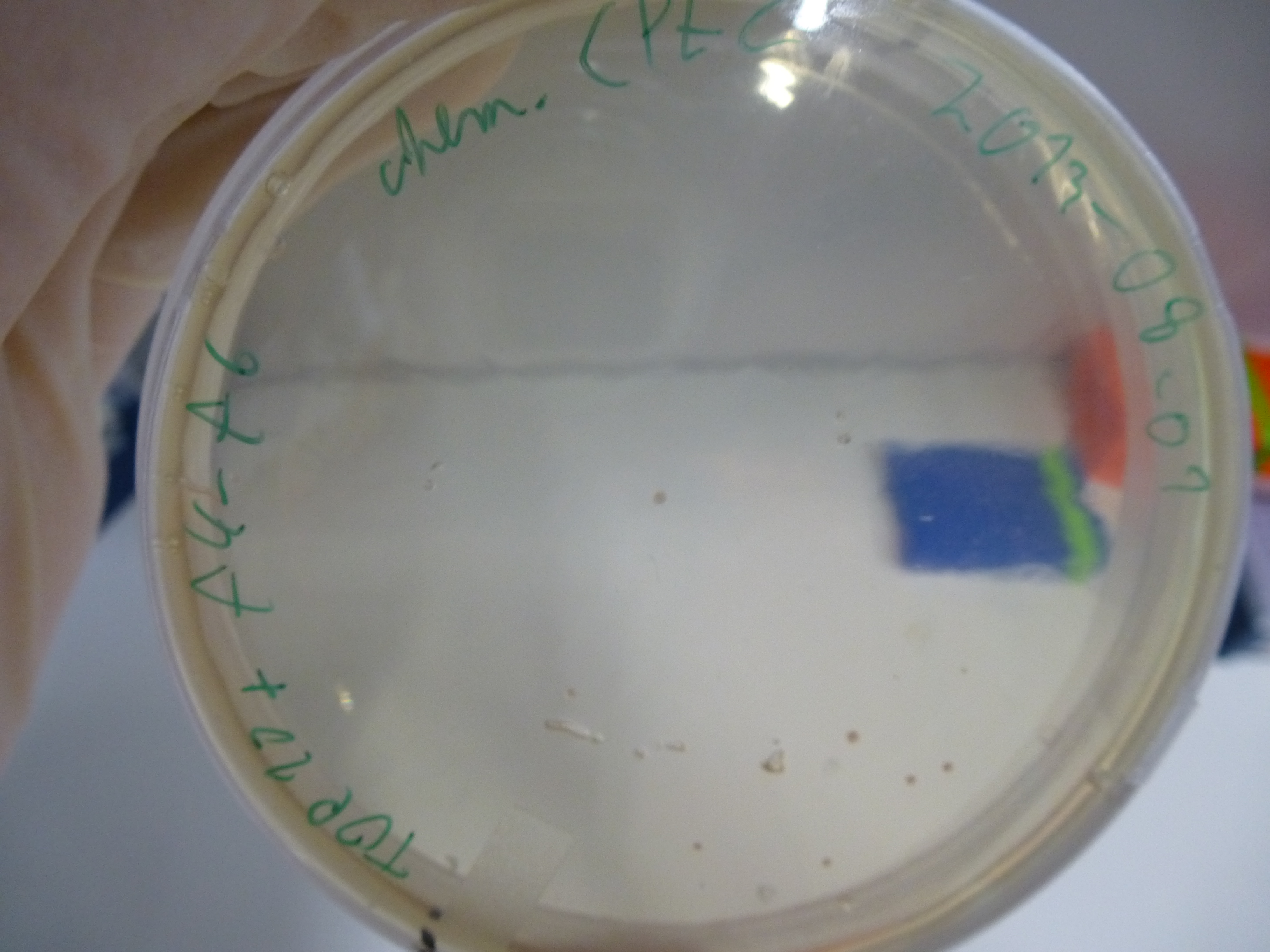
CPEC Assembly of Tripeptide-I-NRPS
- DO ALL WORK ON ICE!!
- mix fragment - DNA aequimolarly --> 10 µl
- add 10 µl of Phusion flash master mix
| Cycles | temperature [°C] | Time [min:s]
|
| 1 | 98 | 0:05
|
| 10 | 98 | 0:01
|
| 55 | 0:05
|
| 72 | 2:00
|
| 1 | 10 | inf
|
- split up 20 µl into:
- 5 µl for chemical transformation
- 10 µl for isopropanol / ethanol DNA-precipitation
- add 10 µl ddH2O to the remaining 5 µl
- electroporate 1 µl
- electroporate 14µl
Gibson Assembly Results
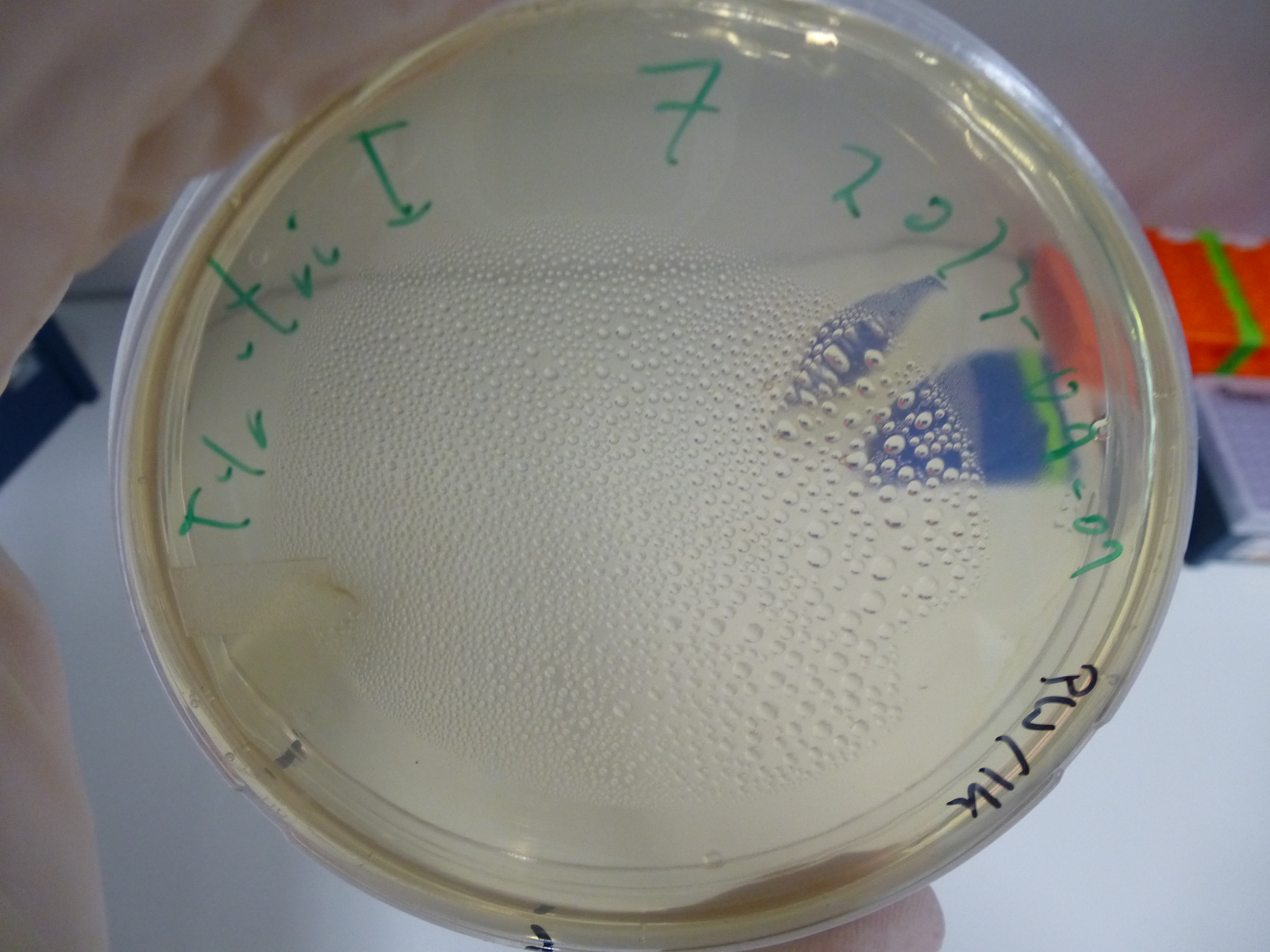
- evaluation of the plates obtained by electroporation our first assembly our first assembly
- picking of colonies from positive plates
- 6 colonies were picked: 3 from plate 5 and 3 from plate 6
- plates:
- 1: Cloning with CPEC - Electroporation with cleaned up DNA
- 2: Cloning with CPEC - Electroporation with 1µl DNA + H2O
- 3: Cloning with CPEC - Electroporation with 14µl DNA + H2O
- 4: Cloning with Gibson - Electroporation with cleaned up DNA
- 5: Cloning with Gibson - Electroporation with 1µl DNA + H2O
- 6: Cloning with Gibson - Electroporation with 14µl DNA + H2O
- 7: Negative Control - Electroporation with H2O
- 8: Cloning with CPEC - Chemical Transformation
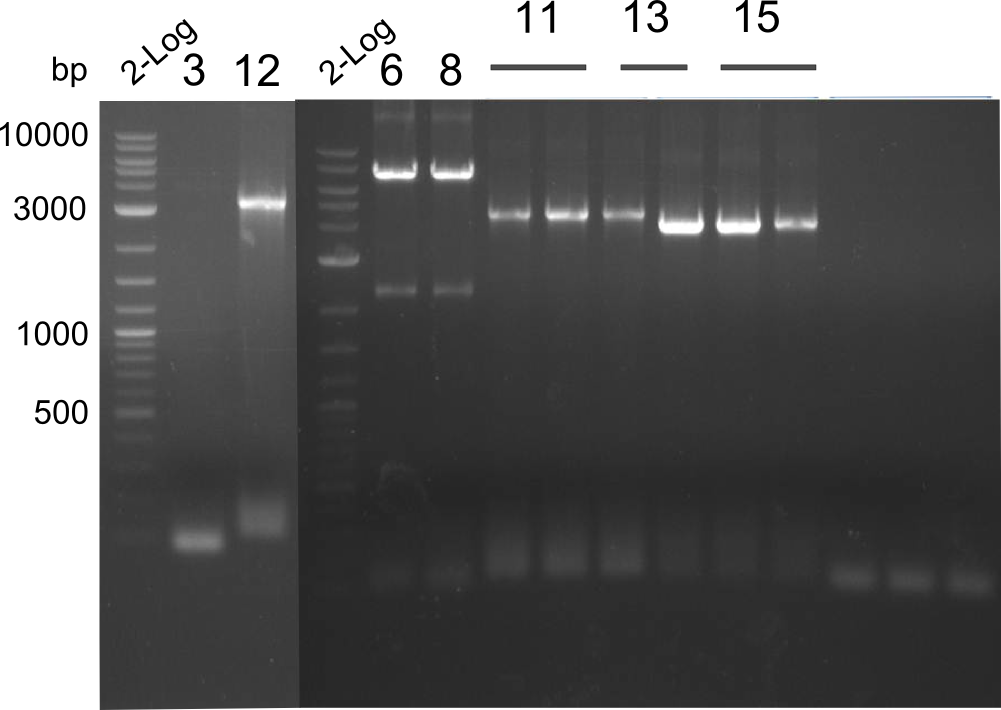
PCR of fragments 3, 12, 6, 8, 11, 13 & 15 - the latter three were conducted with 3 samples each in order to gain more yield
We noticed that we mixed up fragments 1 and 4 thus neither the Gibson nor the CPEC could have worked. We are going to repeat this step next week
Amplifications Round II
Fragment 3
3 IK12,15
| what | µl
|
| fw primer | 2
|
| rv primer | 2
|
| Bacillus | 1
|
| Phusion 2x Master mix | 10
|
| DMSO | 1
|
| ddH20 | 4
|
| Cycles | temperature [°C] | Time [min:s]
|
| 1 | 98 | 2:00
|
| 35 | 98 | 0:01
|
| 65 | 0:05
|
| 72 | 1:00
|
| 1 | 72 | 3:00
|
| 1 | 12 | inf
|
Fragment 6
primer: IK18 & IK12
| what | µl
|
| fw primer | 2
|
| rv primer | 2
|
| Bacillus | 1
|
| Q5 2x Master mix | 10
|
| ddH20 | 5
|
| Cycles | temperature [°C] | Time [min:s]
|
| 1 | 98 | 2:00
|
| 35 | 98 | 0:05
|
| 66 | 0:15
|
| 72 | 3:00
|
| 1 | 72 | 10:00
|
| 1 | 12 | inf
|
Fragment 8
primer: IK20 & IK12
| what | µl
|
| fw primer | 2
|
| rv primer | 2
|
| Bacillus | 1
|
| Q5 2x Master mix | 10
|
| ddH20 | 5
|
| Cycles | temperature [°C] | Time [min:s]
|
| 1 | 98 | 2:00
|
| 35 | 98 | 0:05
|
| 66 | 0:15
|
| 72 | 3:00
|
| 1 | 72 | 10:00
|
| 1 | 12 | inf
|
Fragment 12
12 PW09,10
| what | µl
|
| fw primer | 2
|
| rv primer | 2
|
| Bacillus | 1
|
| Phusion Flash 2x Master mix | 10
|
| DMSO | 1
|
| ddH20 | 4
|
| Cycles | temperature [°C] | Time [min:s]
|
| 1 | 98 | 2:00
|
| 35 | 98 | 0:01
|
| 65 | 0:05
|
| 72 | 1:00
|
| 1 | 72 | 3:00
|
| 1 | 12 | inf
|
Amplifications Round III
- Reamplification of fragments 3, 6, 8, 11, 12, 13 & 15
- Primers used:
- fragment 3: IK15 & IK12
- fragment 6: IK18 & IK12
- fragment 8: IK20 & IK12
- fragment 11: PW07 & PW08
- fragment 12: PW09 & PW10
- fragment 13: PW11 & IK12
- fragment 15: PW13 & IK12
OPTIMIZATION FOR FRAGMENTS 3 & 15 NEEDED, all other fragments obtained
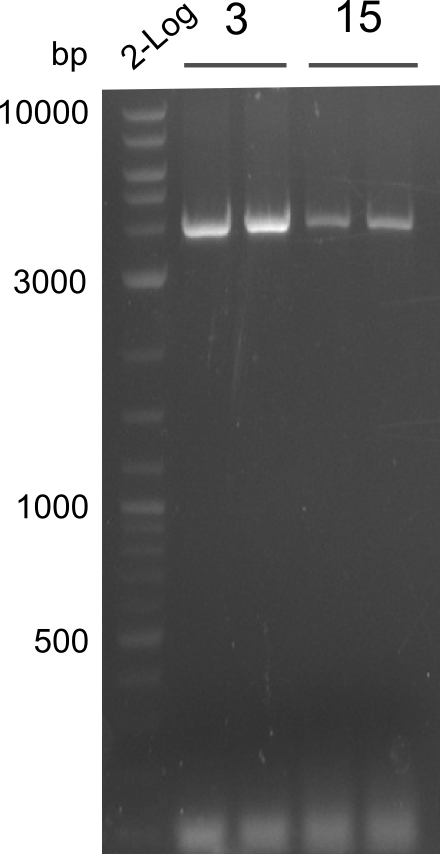
PCR of fragments 3 and 15 for optimization. Phusion Flash Touchdown PCR was used on 02.08.13. Gel was run and picture taken on 03.08.13
- PCRs of Fragments 3 and 15 with phusion flash. T100.
fragment 3: IK15+IK12
fragments 15: IK12+PW13
| what | µl
|
| fw primer | 2
|
| rv primer | 2
|
| Bacillus | 1
|
| Phusion 2x Master mix | 10
|
| DMSO | 1
|
| ddH20 | 4
|
| Cycles | temperature [°C] | Time [s]
|
| 1 | 98 | 2:00
|
| 12 | 98 | 0:05
|
| 66↓0.5°C | 0:05
|
| 72 | 2:30
|
| 23 | 98 | 0:05
|
| 64 | 0:10
|
| 72 | 2:30
|
| 1 | 72 | 10:00
|
| 1 | 10 | inf
|
- gel-extraction of fragments 6, 8, 11, 12 & 13 using long fragment kit
DNA Concentration Measurement
- Quantification Gel of Gibson Assembly Colony Minipreps and fragments 6,8,11,12,13
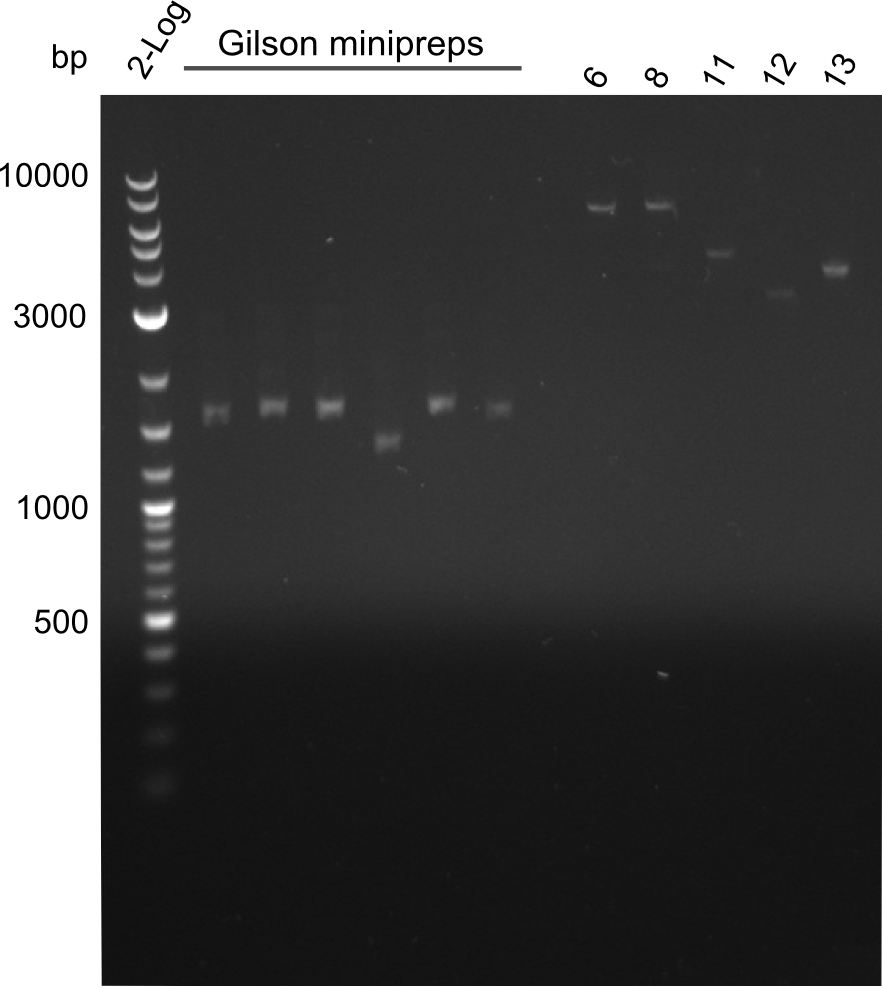
fragments 6, 8, 11, 12 and 13 were extracted from gel with long fragment kit. The minipreps of the Gibson transformants contain plasmids that are way too low - the reason for this is that primers for fragments 1 and 4 were mixed up
| fragment | concentration [ng/µl]
|
| 6 | 6.5
|
| 8 | 5
|
| 11 | 3
|
| 12 | 2
|
| 13 | 7
|
- Gel extraction of fragments 3, 15 and the two unknown samples in the freezer
- Quantification gel of those two fragments
- unfortunately the quantification gel did not show any usable concentrations
- --> reamplification of fragments 3 & 15 - and, as yield was low of fragments 11 & 12
Fragment 3
fragment 3: IK15+IK12
fragments 15: IK12+PW13
| what | µl
|
| fw primer | 2
|
| rv primer | 2
|
| Bacillus | 1
|
| Phusion Flash 2x Master mix | 10
|
| DMSO | 1
|
| ddH20 | 4
|
| Cycles | temperature [°C] | Time [s]
|
| 1 | 98 | 2:00
|
| 12 | 98 | 0:05
|
| 66↓0.5°C | 0:05
|
| 72 | 2:30
|
| 23 | 98 | 0:05
|
| 64 | 0:10
|
| 72 | 2:30
|
| 1 | 72 | 10:00
|
| 1 | 10 | inf
|
Fragment 11
11 PW07,08
| what | µl
|
| fw primer | 2
|
| rv primer | 2
|
| Bacillus | 1
|
| Q5 2x Master mix | 10
|
| ddH20 | 5
|
| Cycles | temperature [°C] | Time [min:s]
|
| 1 | 98 | 2:00
|
| 35 | 98 | 0:05
|
| 69 | 0:15
|
| 72 | 2:30
|
| 1 | 72 | 10:00
|
| 1 | 12 | inf
|
Fragment 12
12 PW09,10
| what | µl
|
| fw primer | 2
|
| rv primer | 2
|
| Bacillus | 1
|
| Phusion Flash 2x Master mix | 10
|
| DMSO | 1
|
| ddH20 | 4
|
| Cycles | temperature [°C] | Time [min:s]
|
| 1 | 98 | 2:00
|
| 35 | 98 | 0:01
|
| 65 | 0:05
|
| 72 | 1:00
|
| 1 | 72 | 3:00
|
| 1 | 12 | inf
|
Fragment 15
fragments 15: IK12+PW13
| what | µl
|
| fw primer | 2
|
| rv primer | 2
|
| Bacillus | 1
|
| Phusion Flash 2x Master mix | 10
|
| DMSO | 1
|
| ddH20 | 4
|
| Cycles | temperature [°C] | Time [s]
|
| 1 | 98 | 2:00
|
| 12 | 98 | 0:05
|
| 66↓0.5°C | 0:05
|
| 72 | 2:30
|
| 23 | 98 | 0:05
|
| 64 | 0:10
|
| 72 | 2:30
|
| 1 | 72 | 10:00
|
| 1 | 10 | inf
|
Results Amplification Round III
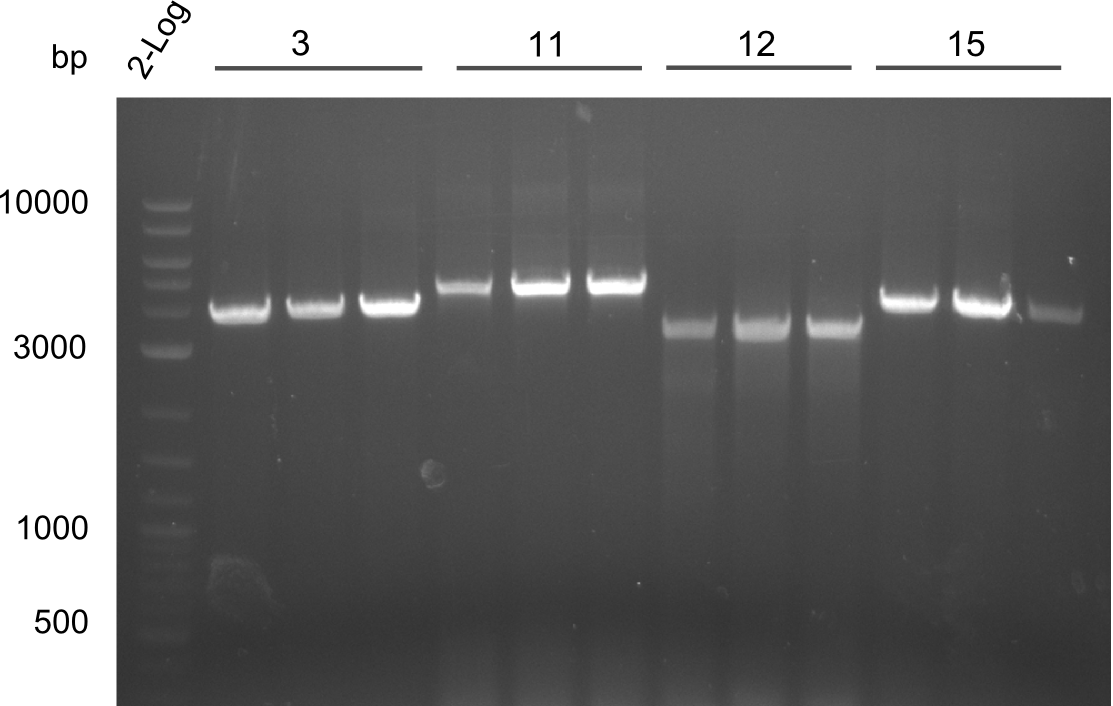
PCR of fragments 3, 11, 12, 13. All lanes were bright and have the right size.
 "
"