Team:Heidelberg/Tyrocidine week18 ms
From 2013.igem.org
Contents |
Final validation of BAP-I-cells and Mass-Spectrometry
SDS-PAGEs of positive BAP-I-cells (Dipeptide & Tripeptide I)
As the SDS-PAGE carried out the week before was unfortunately not successful (no bands were showing up at all), we concluded that the use of another loading buffer, in which the samples are boiled would be useful.
Recipe for loading buffer
The new recipe is as follows:
- 800 µl ddH20
- 250 µl 0.5M Tris-HCl (pH = ~7.3) (i.e. dissolve 302.5 mg Tris in 5 ml and bring to appropriate pH with HCl)
- 400 µl Glycerol 50%
- 400 µl SDS 10%
- 100 µl β-Mercaptoethanol 99%
- bromophenol-blue
Procedure
- Take 1 ml culture in 1.5 ml Eppi
- centrifuge at 13,000 rpm for 10 minutes
- discard supernatant
- resuspend pellet in 100 µl loading buffer (eventually OD-normalize amount of loading buffer)
- boil with closed lids at 98°C for at least 10 minutes under the hood
- cool down (or freeze samples and thaw them again)
- centrifuge at 13,000 rpm for 10 minutes
- load max. 25 µl in SDS-Gel
- use 10 µl BSA-Standard
- let run for 1 hour at 180 V
Results
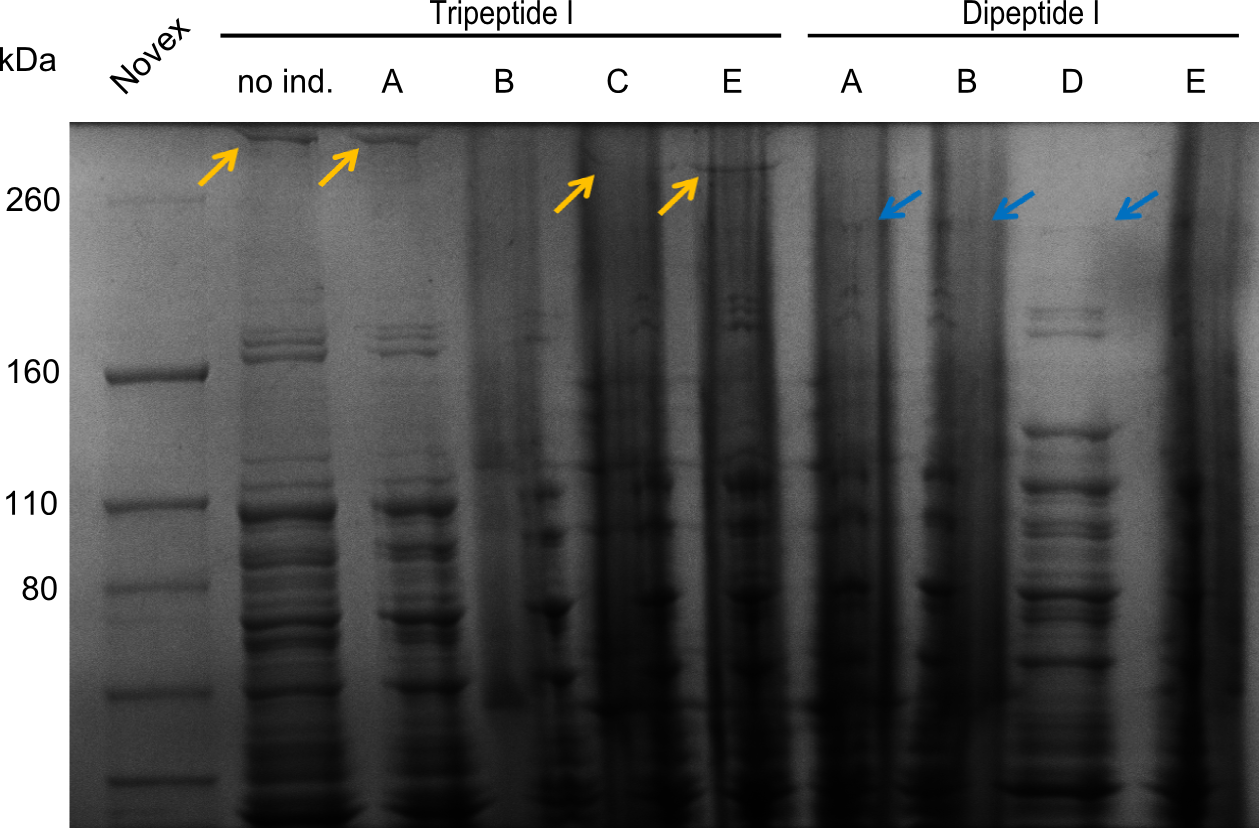
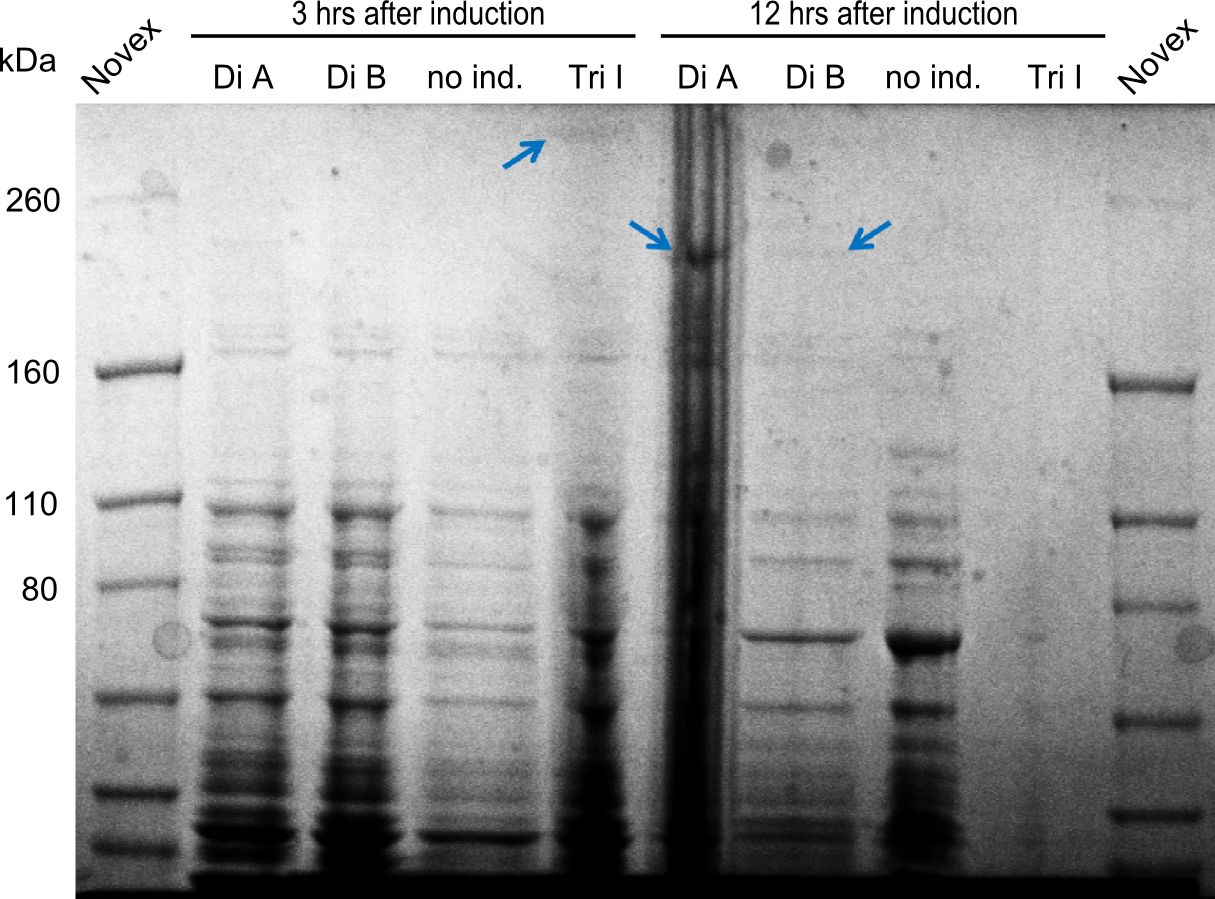
Two seperate SDS-PAGEs were conducted using the protocol described above.
blue arrows indicate the desired synthetases. For the synthetases of Dipeptide and the Tripeptide-I 212 kDa and 380 kDa bands were expected. The golden arrows indicate a possible lane for the NRPS. For the 380 kDa construct, it is not 100% clear, where one should find it on the gel.
Screening for positive Tetrapeptide-I-colonies
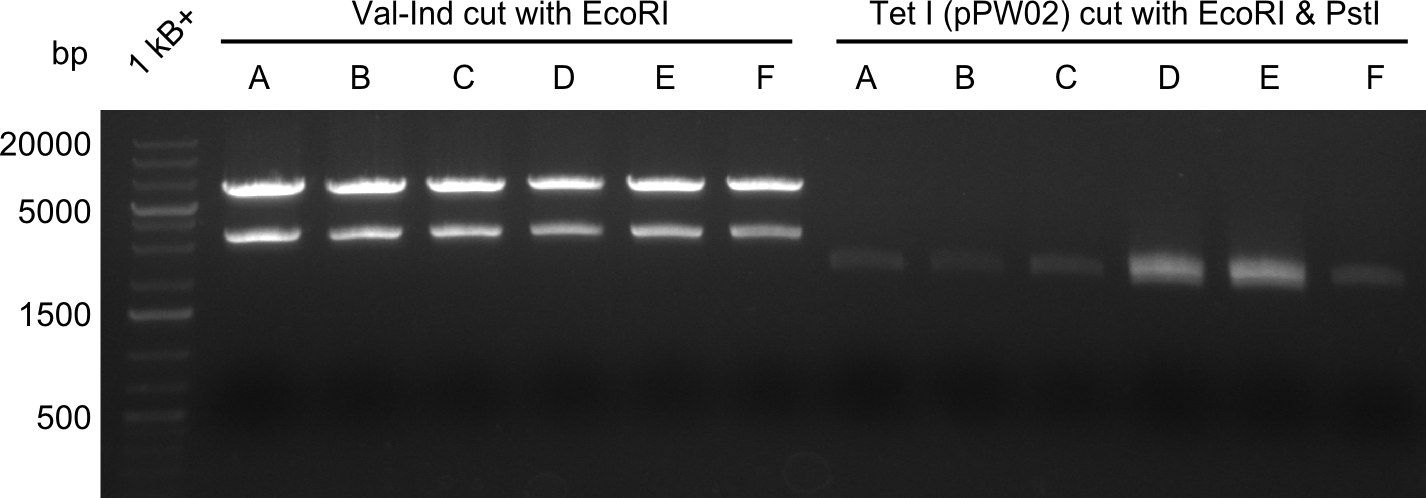
As we did not succeed in finding a positive colony in BAP-I-cells, we decided to continue screening for positive colonies. This however proved to be ineffective as one can see in the image below. All colonies that were grown in liquid culture, prepped and digested with EcoRI and PstI are negative for the insert.
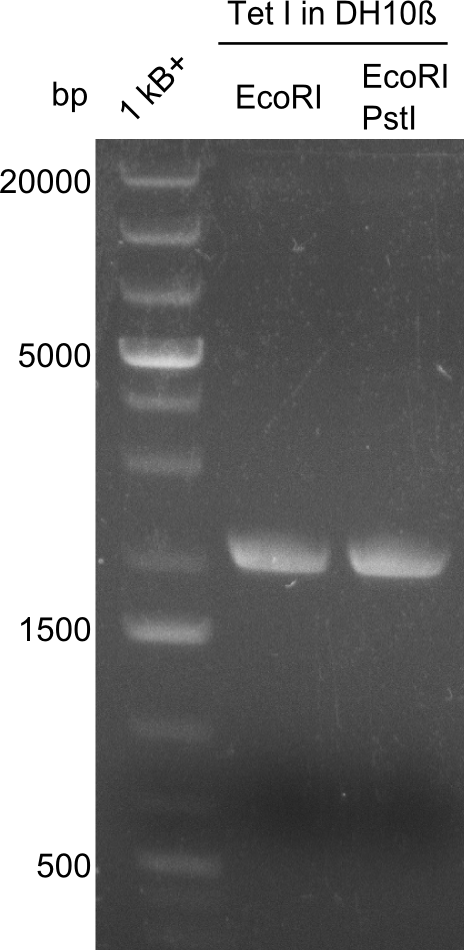
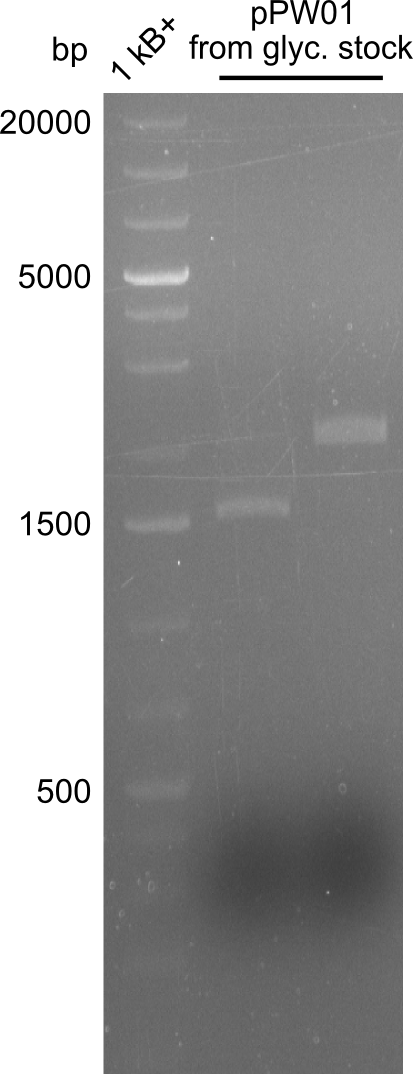
So far, restriction digests were neagtive for Tetrapeptide-I (pPW01) transformed cells, i.e. only the backbone was detectable. For whatever reasons, we had somehow lost the positive plasmids (or couldn't transform them in chemically competent BAP-I cells due to their size. We therefore analyzed the glycerol-stocks we had prepared and performed restriction digests on the plasmids obtained after miniprep.
Both glycerol stocks, the one for DH10β (left image), as well as the one for BAP-I (right image) were clearly negative.
As we however have to functional plasmids, we will focus on the analysis of those and abandon our work on the Tetrapeptide-I-construct pPW01.
 "
"