Team:Heidelberg/Tyrocidine week20 interms
From 2013.igem.org
(Difference between revisions)
(Created page with " == Tyrocidine-Indigoidine fusion - extended == As Valin-Indigoidine and its synthetase could be detected by TLC and SDS-PAGE respectively, we decided to increase the number of F...") |
|||
| Line 3: | Line 3: | ||
As Valin-Indigoidine and its synthetase could be detected by TLC and SDS-PAGE respectively, we decided to increase the number of Fusion-constructs in order to test Indigoidine as possible tag for NRPs and their synthesis. Herefore, we aim to build the following 7 NRPs: | As Valin-Indigoidine and its synthetase could be detected by TLC and SDS-PAGE respectively, we decided to increase the number of Fusion-constructs in order to test Indigoidine as possible tag for NRPs and their synthesis. Herefore, we aim to build the following 7 NRPs: | ||
| - | [[File: | + | [[File:Heidelberg_Tyc-Ind Fusion_extended.jpeg|1000px|center|thumb]] |
=== Amplifications === | === Amplifications === | ||
| Line 484: | Line 484: | ||
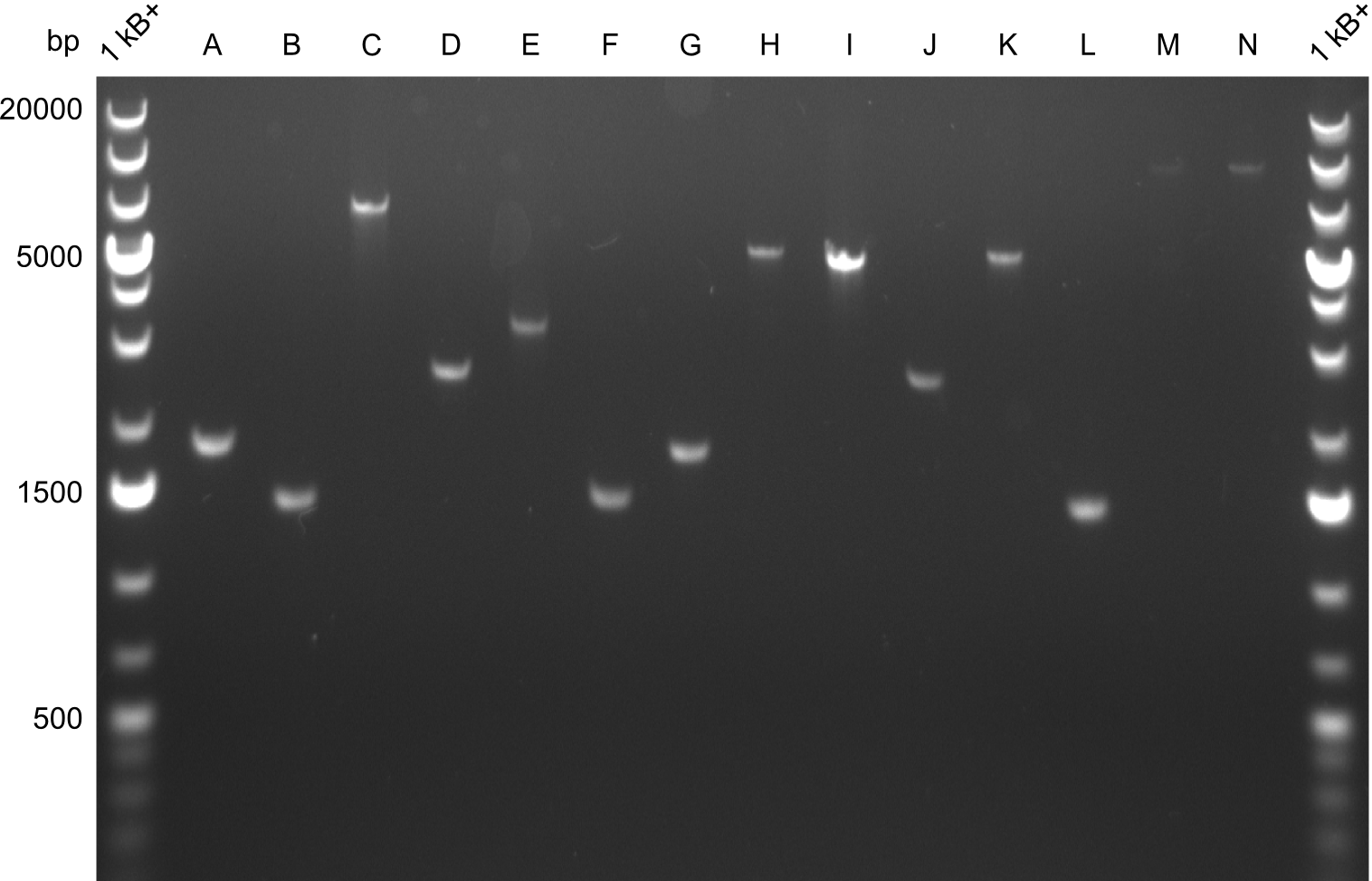
==== Gel-Pictures ==== | ==== Gel-Pictures ==== | ||
| - | [[File: | + | [[File:Heidelberg_TIFEXT_ABDEFGHJKLMN_PCR_1st.png|300px]] |
| - | [[File: | + | [[File:Heidelberg_TIFEXT_CCIIMMNN_PCR_2nd.png|420px]] |
| - | [[File: | + | [[File:Heidelberg_TIFEXT_CC_PCR_3rd.png|190px|The PCR for fragment C was repeated after it was completely used in Gibson assemblies]] |
==== Quantification-Gel ==== | ==== Quantification-Gel ==== | ||
| - | [[File: | + | [[File:Heidelberg_TIFEXT_all_fragments_QUANT_1st.png|300px]] |
=== Gibson Assembly === | === Gibson Assembly === | ||
| Line 596: | Line 596: | ||
==== Colony-PCR ==== | ==== Colony-PCR ==== | ||
<gallery> | <gallery> | ||
| - | File: | + | File:Heidelberg_COLPCR_09_11_12_Tyc-part.png|Screening for the Tyrocidine-part with VF2 and PW14 |
| - | File: | + | File:Heidelberg_COLPCR_09_11_12_Ind-part.png|Screening for the Indigoidine-part with VR and KH05 |
</gallery> | </gallery> | ||
| Line 603: | Line 603: | ||
<gallery> | <gallery> | ||
| - | File: | + | File:Heidelberg_COLPCR_06_09_10_11_12_Tyc-part.png|Screening for the Tyrocidine-part with VF2 and PW14]] |
| - | File: | + | File:Heidelberg_COLPCR_06_09_10_11_12_Ind-part.png|Screening for the Indigoidine-part with VR and KH05]] |
</gallery> | </gallery> | ||
| Line 615: | Line 615: | ||
<gallery> | <gallery> | ||
| - | File: | + | File:Heidelberg_EcoRI_06_10_11.png|digest with EcoRI after 20 min on gel]] |
| - | File: | + | File:Heidelberg_EcoRI_06_10_11_LONG.png|digest with EcoRI after 40 min on gel]] |
</gallery> | </gallery> | ||
After only 20 minutes of running, the bands were not yet clearly separated. Waiting for another 20 minutes lead to a interpretable result. | After only 20 minutes of running, the bands were not yet clearly separated. Waiting for another 20 minutes lead to a interpretable result. | ||
Revision as of 17:08, 4 October 2013
Contents |
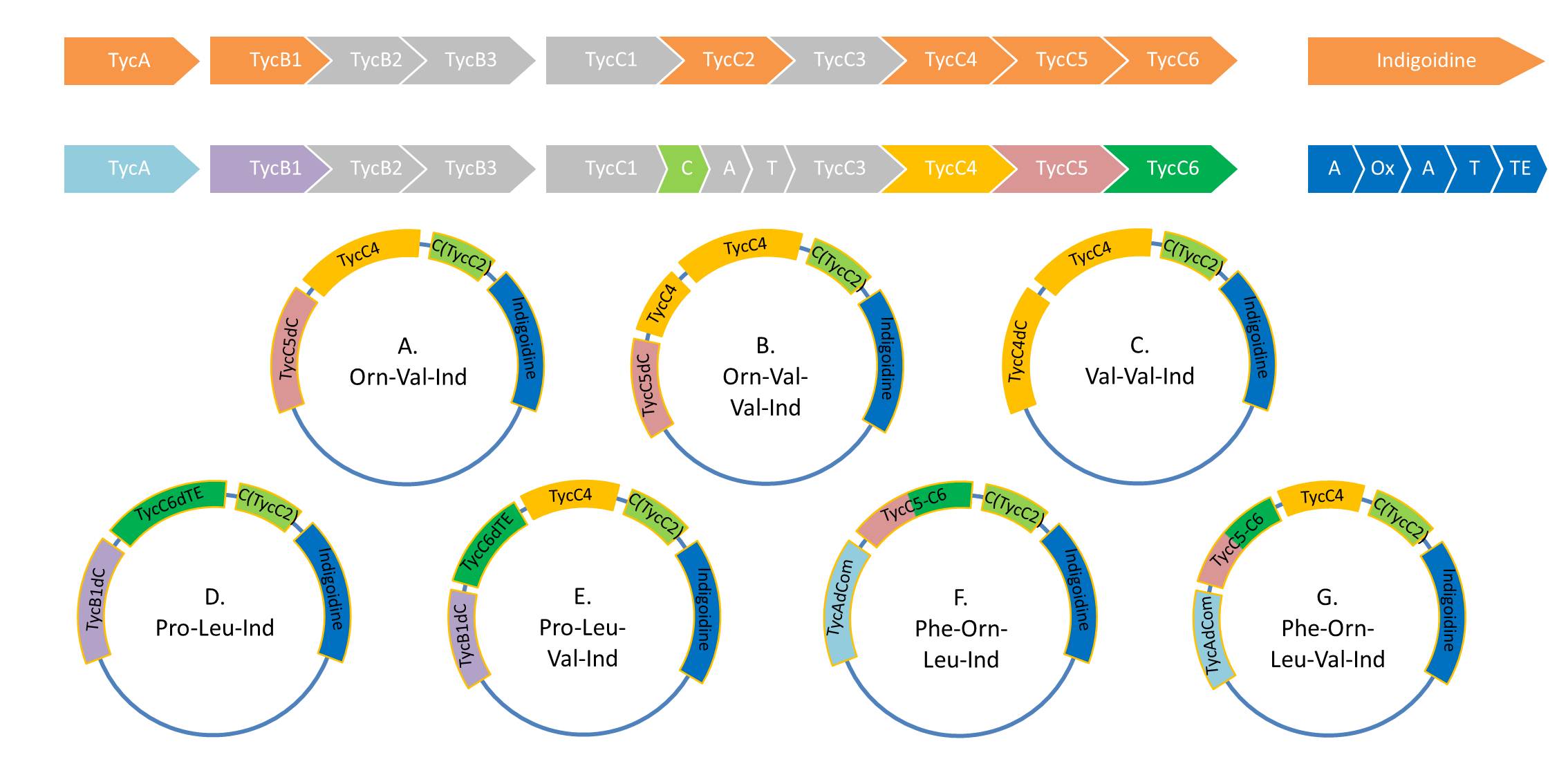
Tyrocidine-Indigoidine fusion - extended
As Valin-Indigoidine and its synthetase could be detected by TLC and SDS-PAGE respectively, we decided to increase the number of Fusion-constructs in order to test Indigoidine as possible tag for NRPs and their synthesis. Herefore, we aim to build the following 7 NRPs:
Amplifications
Protocols
A
| what | µl |
|---|---|
| pIK04 | 1 |
| PW26 | 2 |
| PW27 | 2 |
| Phusion Flash 2x Master Mix | 10 |
| ddH20 | 5 |
| Cycles | temperature [°C] | Time [min:s] |
|---|---|---|
| 1 | 98 | 0:05 |
| 35 | 98 | 0:05 |
| 66 | 0:10 | |
| 72 | 0:50 | |
| 1 | 72 | 10:00 |
| 1 | 10 | inf |
B
| what | µl |
|---|---|
| B. parabrevis | 1 |
| PW28 | 2 |
| PW29 | 2 |
| Phusion Flash 2x Master Mix | 10 |
| ddH20 | 5 |
| Cycles | temperature [°C] | Time [min:s] |
|---|---|---|
| 1 | 98 | 0:05 |
| 35 | 98 | 0:05 |
| 58 | 0:10 | |
| 72 | 0:30 | |
| 1 | 72 | 10:00 |
| 1 | 10 | inf |
C
| what | µl |
|---|---|
| pPW05 | 1 |
| PW30 | 2 |
| PW16 | 2 |
| Phusion Flash 2x Master Mix | 10 |
| ddH20 | 5 |
| Cycles | temperature [°C] | Time [min:s] |
|---|---|---|
| 1 | 98 | 0:05 |
| 35 | 98 | 0:05 |
| 64 | 0:10 | |
| 72 | 2:00 | |
| 1 | 72 | 10:00 |
| 1 | 10 | inf |
D
| what | µl |
|---|---|
| pIK04 | 1 |
| PW37 | 2 |
| PW23 | 2 |
| Phusion Flash 2x Master Mix | 10 |
| ddH20 | 5 |
| Cycles | temperature [°C] | Time [min:s] |
|---|---|---|
| 1 | 98 | 0:05 |
| 35 | 98 | 0:05 |
| 66 | 0:10 | |
| 72 | 0:50 | |
| 1 | 72 | 10:00 |
| 1 | 10 | inf |
E
| what | µl |
|---|---|
| B. parabrevis | 1 |
| PW28 | 2 |
| PW31 | 2 |
| Phusion Flash 2x Master Mix | 10 |
| ddH20 | 5 |
| Cycles | temperature [°C] | Time [min:s] |
|---|---|---|
| 1 | 98 | 0:05 |
| 35 | 98 | 0:05 |
| 63 | 0:10 | |
| 72 | 1:00 | |
| 1 | 72 | 10:00 |
| 1 | 10 | inf |
F
| what | µl |
|---|---|
| B. parabrevis | 1 |
| PW32 | 2 |
| PW29 | 2 |
| Phusion Flash 2x Master Mix | 10 |
| ddH20 | 5 |
| Cycles | temperature [°C] | Time [min:s] |
|---|---|---|
| 1 | 98 | 0:05 |
| 35 | 98 | 0:05 |
| 58 | 0:10 | |
| 72 | 0:30 | |
| 1 | 72 | 10:00 |
| 1 | 10 | inf |
G
| what | µl |
|---|---|
| pPW05 | 1 |
| PW19 | 2 |
| PW31 | 2 |
| Phusion Flash 2x Master Mix | 10 |
| ddH20 | 5 |
| Cycles | temperature [°C] | Time [min:s] |
|---|---|---|
| 1 | 98 | 0:05 |
| 35 | 98 | 0:05 |
| 66 | 0:10 | |
| 72 | 0:50 | |
| 1 | 72 | 10:00 |
| 1 | 10 | inf |
H
| what | µl |
|---|---|
| pIK03 | 1 |
| IK13 | 2 |
| PW33 | 2 |
| Phusion Flash 2x Master Mix | 10 |
| ddH20 | 5 |
| Cycles | temperature [°C] | Time [min:s] |
|---|---|---|
| 1 | 98 | 0:05 |
| 35 | 98 | 0:05 |
| 65 | 0:10 | |
| 72 | 1:40 | |
| 1 | 72 | 10:00 |
| 1 | 10 | inf |
I
| what | µl |
|---|---|
| pPW05 | 1 |
| PW34 | 2 |
| PW16 | 2 |
| Phusion Flash 2x Master Mix | 10 |
| ddH20 | 5 |
| Cycles | temperature [°C] | Time [min:s] |
|---|---|---|
| 1 | 98 | 0:05 |
| 35 | 98 | 0:05 |
| 60 | 0:10 | |
| 72 | 1:40 | |
| 1 | 72 | 10:00 |
| 1 | 10 | inf |
J
| what | µl |
|---|---|
| pIK04 | 1 |
| IK23 | 2 |
| PW23 | 2 |
| Phusion Flash 2x Master Mix | 10 |
| ddH20 | 5 |
| Cycles | temperature [°C] | Time [min:s] |
|---|---|---|
| 1 | 98 | 0:05 |
| 35 | 98 | 0:05 |
| 66 | 0:10 | |
| 72 | 0:50 | |
| 1 | 72 | 10:00 |
| 1 | 10 | inf |
K
| what | µl |
|---|---|
| pIK03 | 1 |
| IK13 | 2 |
| PW35 | 2 |
| Phusion Flash 2x Master Mix | 10 |
| ddH20 | 5 |
| Cycles | temperature [°C] | Time [min:s] |
|---|---|---|
| 1 | 98 | 0:05 |
| 35 | 98 | 0:05 |
| 65 | 0:10 | |
| 72 | 1:40 | |
| 1 | 72 | 10:00 |
| 1 | 10 | inf |
L
| what | µl |
|---|---|
| B. parabrevis | 1 |
| PW36 | 2 |
| PW29 | 2 |
| Phusion Flash 2x Master Mix | 10 |
| ddH20 | 5 |
| Cycles | temperature [°C] | Time [min:s] |
|---|---|---|
| 1 | 98 | 0:05 |
| 35 | 98 | 0:05 |
| 58 | 0:10 | |
| 72 | 0:30 | |
| 1 | 72 | 10:00 |
| 1 | 10 | inf |
M
| what | µl |
|---|---|
| pIK04 | 1 |
| IK16 | 2 |
| PW33 | 2 |
| Phusion Flash 2x Master Mix | 10 |
| ddH20 | 5 |
| Cycles | temperature [°C] | Time [min:s] |
|---|---|---|
| 1 | 98 | 0:05 |
| 35 | 98 | 0:05 |
| 65 | 0:10 | |
| 72 | 3:10 | |
| 1 | 72 | 10:00 |
| 1 | 10 | inf |
N
| what | µl |
|---|---|
| pIK04 | 1 |
| IK16 | 2 |
| PW35 | 2 |
| Phusion Flash 2x Master Mix | 10 |
| ddH20 | 5 |
| Cycles | temperature [°C] | Time [min:s] |
|---|---|---|
| 1 | 98 | 0:05 |
| 35 | 98 | 0:05 |
| 65 | 0:10 | |
| 72 | 3:10 | |
| 1 | 72 | 10:00 |
| 1 | 10 | inf |
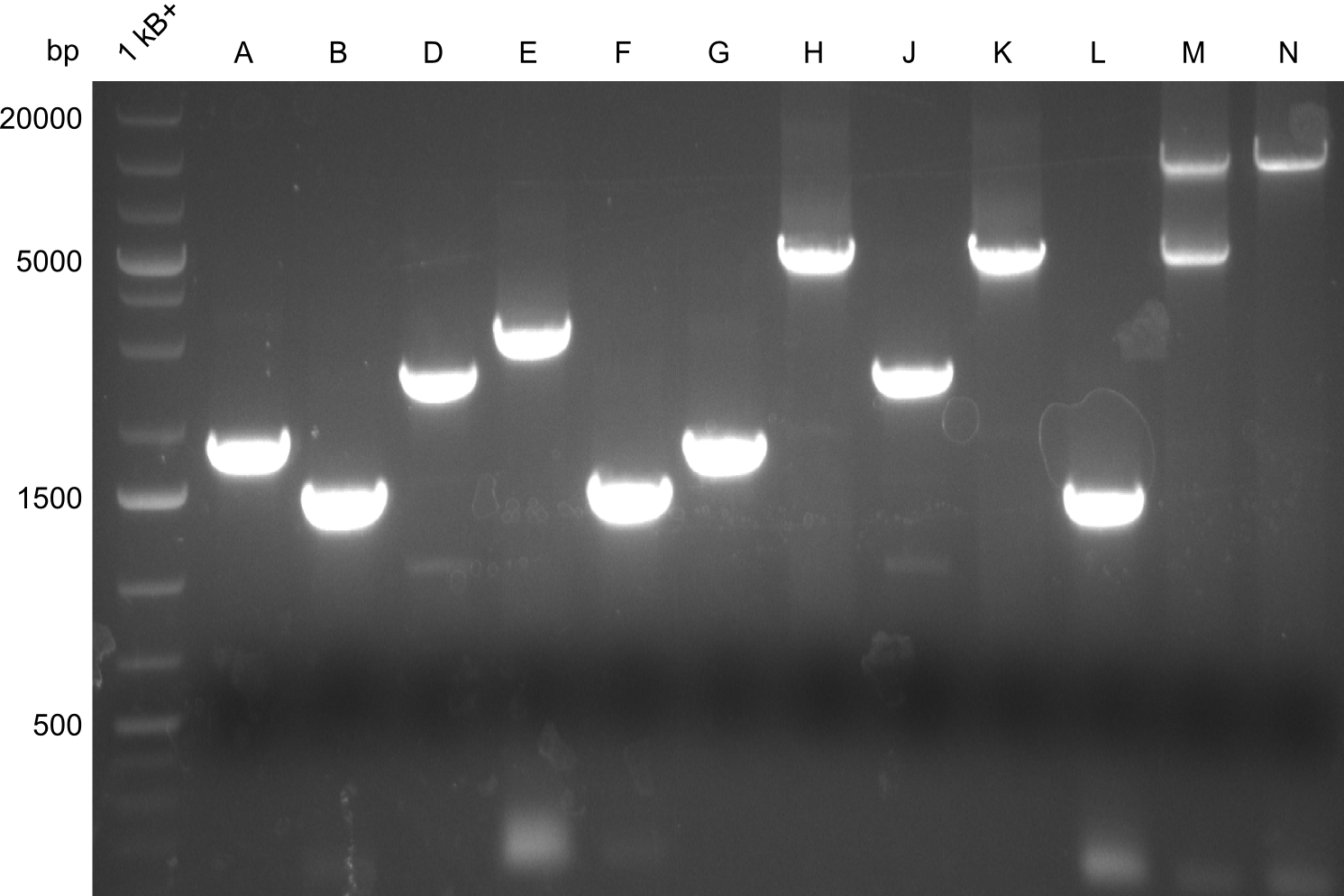
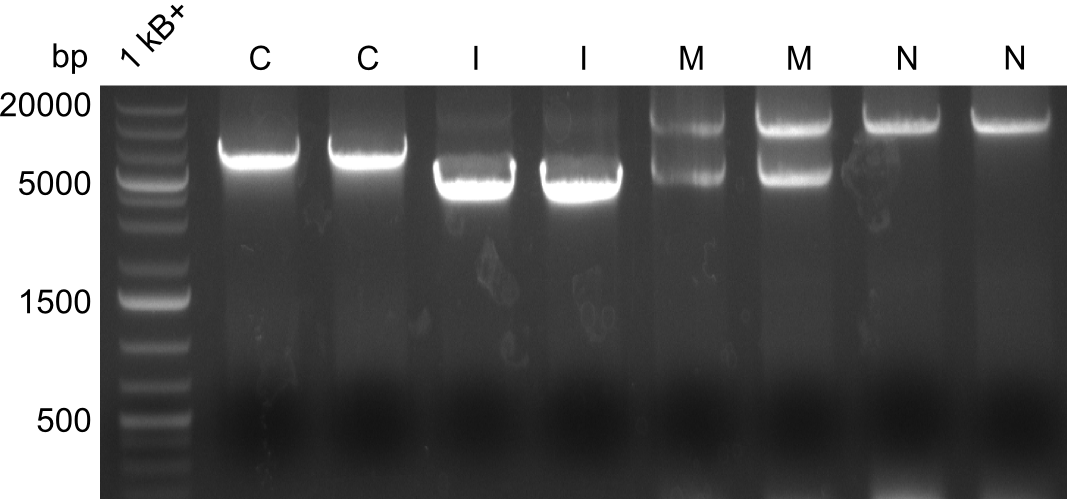
Gel-Pictures
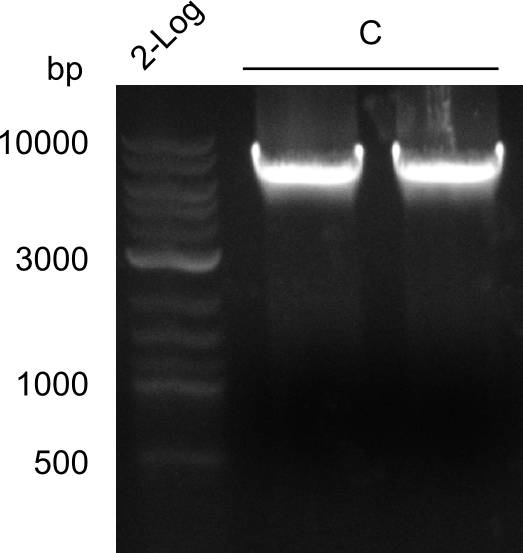
Quantification-Gel
Gibson Assembly
pPW06 (Orn-Val-Ind)
| fragment | concentration [ng/µl] | volume for gibson assembly [µL] |
|---|---|---|
| A | 40 | 1.61 |
| B | 35 | 0.92 |
| C | 30 | 6.97 |
| D | 40 | 0.50 |
pPW07 (Orn-Val-Val-Ind)
| fragment | concentration [ng/µl] | volume for gibson assembly [µL] |
|---|---|---|
| A | 40 | 1.16 |
| C | 30 | 4.32 |
| D | 40 | 0.36 |
| E | 20 | 3.49 |
| F | 35 | 0.66 |
pPW08 (Val-Val-Ind)
| fragment | concentration [ng/µl] | volume for gibson assembly [µL] |
|---|---|---|
| C | 30 | 6.07 |
| F | 35 | 0.93 |
| G | 40 | 1.63 |
| OldG | 15 | 1.36 |
pPW09 (Pro-Leu-Ind)
| fragment | concentration [ng/µl] | volume for gibson assembly [µL] |
|---|---|---|
| H | 20 | 6.35 |
| I | 40 | 3.02 |
| J | 25 | 0.63 |
pPW10 (Pro-Leu-Val-Ind)
| fragment | concentration [ng/µl] | volume for gibson assembly [µL] |
|---|---|---|
| C | 30 | 4.58 |
| J | 20 | 0.66 |
| K | 25 | 4.23 |
| L | 40 | 0.53 |
pPW11 (Phe-Orn-Leu-Ind)
| fragment | concentration [ng/µl] | volume for gibson assembly [µL] |
|---|---|---|
| I | 40 | 0.58 |
| J | 20 | 0.15 |
| M | 5 | 9.27 |
pPW12 (Phe-Orn-Leu-Val-Ind)
| fragment | concentration [ng/µl] | volume for gibson assembly [µL] |
|---|---|---|
| C | 30 | 1.77 |
| J | 20 | 0.26 |
| L | 40 | 0.20 |
| N | 10 | 7.77 |
Cells were transformed with 1 µl and 14 µl 1:2 diluted Gibson-Mix.
Screening for positive transformants
Colony-PCR
First, there were colonies visible for the transformants of pPW09, pPW11 and pPW12. Later colonies for pPW06 and pPW10 followed.
Restriction Digest
Positive samples were digested with restriction digest with EcoRI. The expected sizes of bands were:
- pPW06:
- pPW09:
- pPW10:
- pPW11:
After only 20 minutes of running, the bands were not yet clearly separated. Waiting for another 20 minutes lead to a interpretable result.
 "
"