Team:Heidelberg/Tyrocidine week21 biobrickmod
From 2013.igem.org
(Created page with "== Tyrocidine-Indigoidine-fusion - standardization == === Reassembly === As CPEC-approaches did not work out so far, a reassembly of the fusion constructs was run in parallel. He...") |
|||
| Line 1: | Line 1: | ||
| + | |||
== Tyrocidine-Indigoidine-fusion - standardization == | == Tyrocidine-Indigoidine-fusion - standardization == | ||
=== Reassembly === | === Reassembly === | ||
| Line 50: | Line 51: | ||
<gallery> | <gallery> | ||
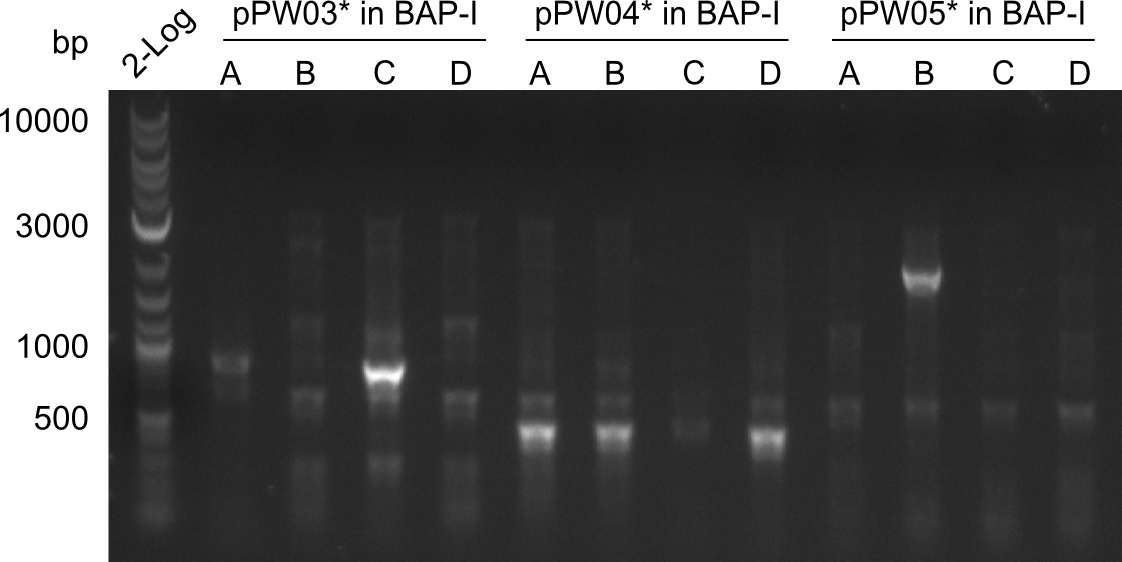
| - | File: | + | File:Heidelberg_COLPCR_1'_2'_3'_Tyc.png|Screening for the Tyrocidine-part in the various colonies picked after electroporation of the Gibson-Mix. |
| - | File: | + | File:Heidelberg_COLPCR_1'_2'_3'_Ind.png|Screening for the Indigoidinde-part in the various colonies picked after electroporation of the Gibson-Mix. |
| - | File: | + | File:Heidelberg_COLPCR_1'_2'_3'_Ind_REPEAT.png|Repetition of the sceening with overnight-cultures. |
</gallery> | </gallery> | ||
| Line 82: | Line 83: | ||
<gallery> | <gallery> | ||
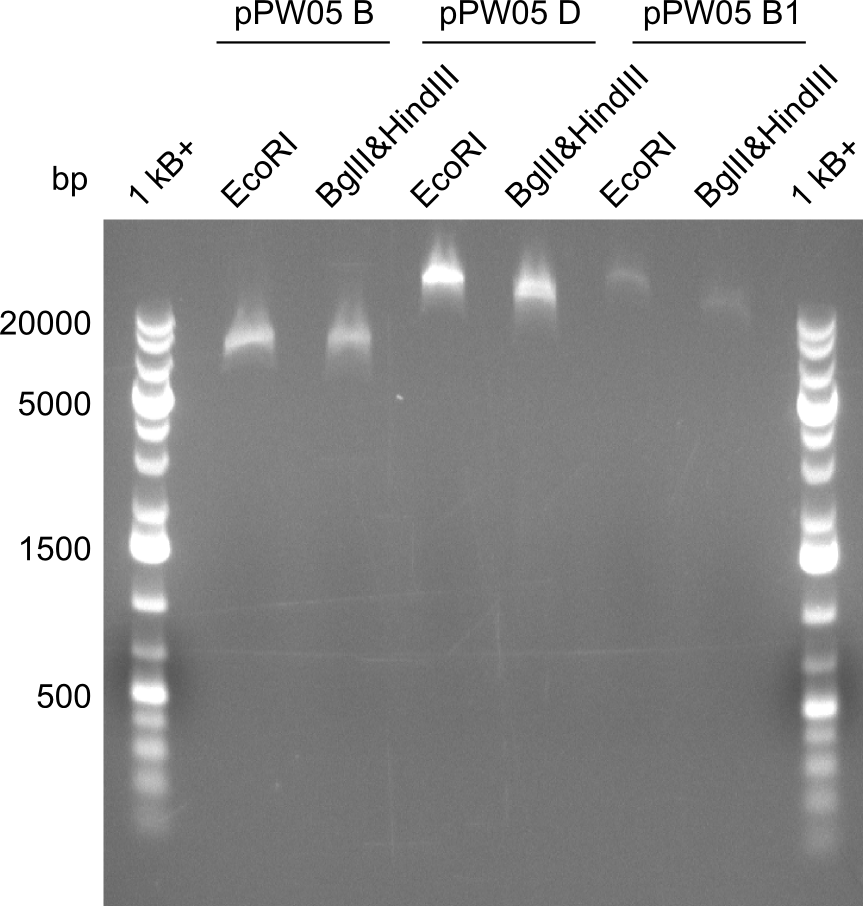
| - | File: | + | File:Heidelberg_RESTR_ECO_BGLHIND_3.png|Restriciton digest with EcoRI and BglII&HindIII |
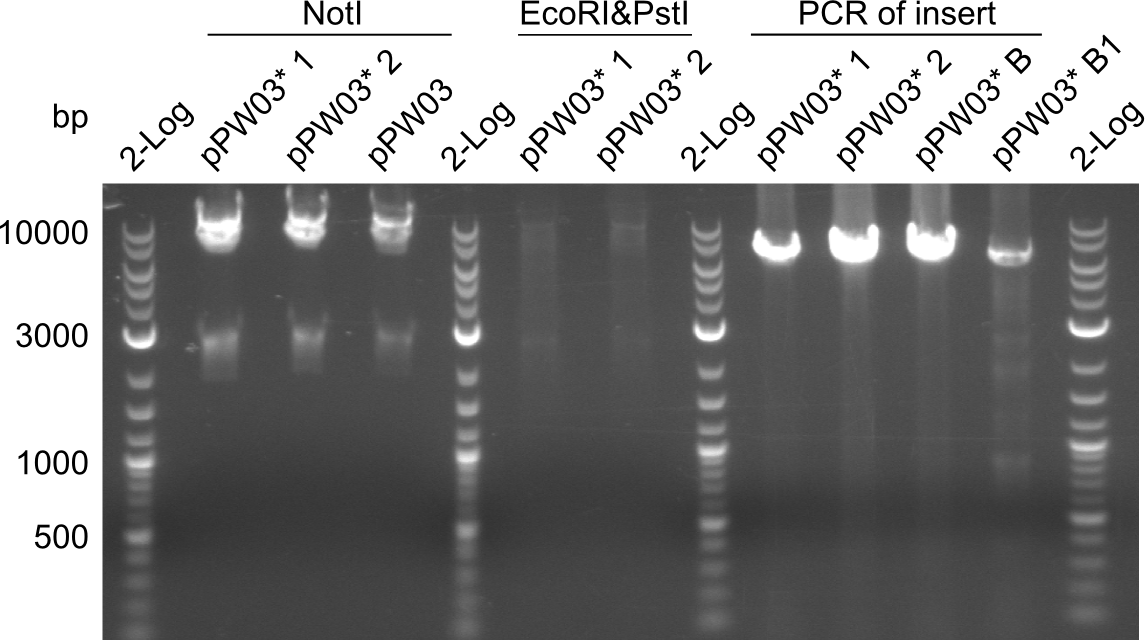
| - | File: | + | File:Heidelberg_RESTR_pPW03_1_2_ECOPST_MFE_ECOHIND.png|Restriction digest with EcoRI&PstI, MfeI and EcoRI&HindIII |
| - | File: | + | File:Heidelberg_RESTR_pPW03_1_2_ECOPST_NOT_PCR.png|Restriction digest with EcoRI&PstI and NotI, PCR of the insert |
</gallery> | </gallery> | ||
| Line 93: | Line 94: | ||
<gallery> | <gallery> | ||
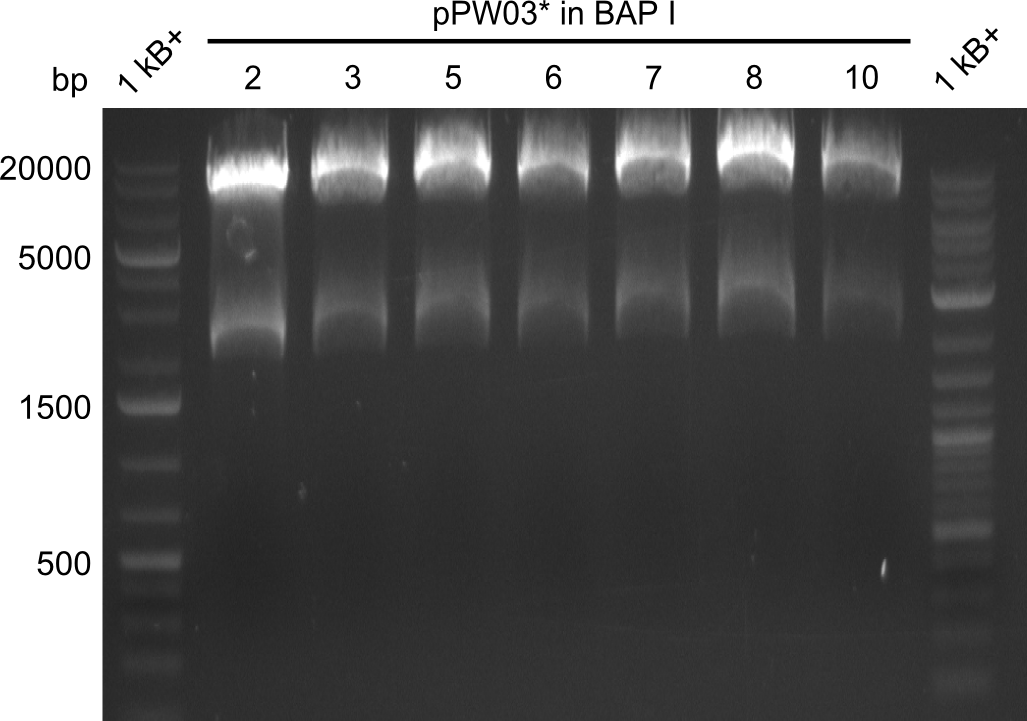
| - | File: | + | File:Heidelberg_RESTR_ECOPST_pPW05'2-10_PARTS.png|Restriction digest with EcoRI and PstI, ran at 135V |
| - | File: | + | File:Heidelberg_RESTR_ECOPST_pPW05'2-10_PARTS_CLEAN.png|Restriction digest purified with isopropanol, ran at 100V |
</gallery> | </gallery> | ||
Again, the pattern of bands was uncommon and unexpected, hence we purified the mixture to remove residual enzyme and cut DNA with isopropanol. Analysing this on a gel that was run at 100V yielded a better result. The obtained bands met our expectations. For validation, a TLC was run with the samples, which again showed the slower migration of Val-Ind. | Again, the pattern of bands was uncommon and unexpected, hence we purified the mixture to remove residual enzyme and cut DNA with isopropanol. Analysing this on a gel that was run at 100V yielded a better result. The obtained bands met our expectations. For validation, a TLC was run with the samples, which again showed the slower migration of Val-Ind. | ||
Finally, we could submit our samples to the registry, as sequencing was positive for all linkers. | Finally, we could submit our samples to the registry, as sequencing was positive for all linkers. | ||
Latest revision as of 17:11, 4 October 2013
Contents |
Tyrocidine-Indigoidine-fusion - standardization
Reassembly
As CPEC-approaches did not work out so far, a reassembly of the fusion constructs was run in parallel. Herefore, a standardized indC-module, that was prepared by the Indigoidine group via CPEC, was used.
The following formulas for gibson assembly were calculated:
pPW03 (Phe-Ind)
| Fragment | Concentration [ng/µl] | Volume for gibson assembly [µL] |
|---|---|---|
| A | 12 | 3.29 |
| B | 8 | 3.95 |
| C | 6 | 2.63 |
| D* | 120 | 0.13 |
pPW04 (Asn-Ind)
| Fragment | Concentration [ng/µl] | Volume for gibson assembly [µL] |
|---|---|---|
| D* | 120 | 0.25 |
| E | 20 | 3.75 |
| F | 20 | 6.00 |
pPW05 (Val-Ind)
| fragment | Concentration [ng/µl] | Volume for gibson assembly [µL] |
|---|---|---|
| D* | 120 | 0.26 |
| G | 15 | 4.21 |
| H | 16 | 3.95 |
| I | 20 | 1.58 |
Results
As shown in the images above, this approach did not lead to the desired result, as mostly colonies that were negative in the colony PCR were growing on the plate. Therefore, we decided to change the Gibson-protocol once more, using less backbone and more indC. For this optimization, we focussed on the Val-Ind-NRPS encoded by pPW05. However, the only positive colony (pPW05* B) was kept for further analysis.
Optimization
With the following optimization:
| Fragment | Concentration [ng/µl] | Volume for gibson assembly [µL] |
|---|---|---|
| D* | 120 | 1.67 |
| G | 15 | 1.33 |
| H | 16 | 5.00 |
| I | 20 | 2.00 |
Results: Only few colonies were growing after electroporation.
Validation
Procedure
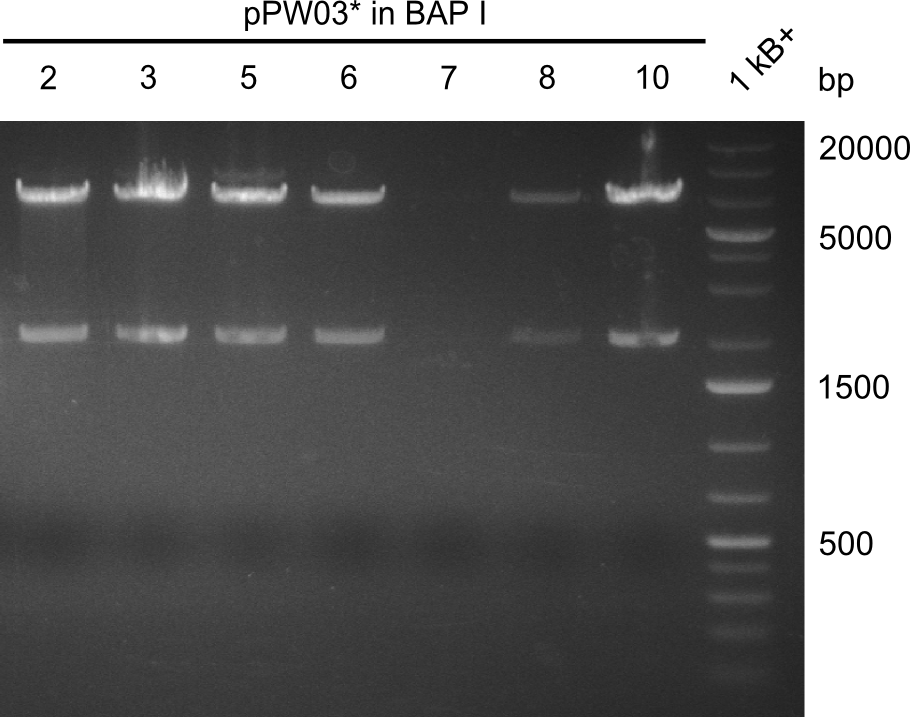
Restriction digests were performed with the so far promising samples. The gels after digest (which were run at 135V) all had strange running patterns, as it seemed as if all of the samples ran too high.
Even though the sequencing was positive for all of those samples, we were concerned because of the unexpected restriction patterns. Therefore, we picked 12 colonies from remaining plates, let them grew overnight, took 2ml for miniprep and induced the remaining 1ml. In addition, we transformed the promising samples, that were already positive in sequencing, into BAP-I. Transformation was also performed for TOP10 and NEB-Turbo cells, which were also co-transformed with a plasmid carrying a PPTase and kanamycin resistance. Instead of plating them and picking colonies, we decided to grow the transformants directly in LB+Cm-selection medium. The next morning, we induced them as well.
Results
There were 7 out of the 12 colonies that turned blue after induction with 1mM IPTG, hence they were miniprepped and digested with EcoRI and PstI. Only half of the volume was loaded on a gel, the other half was purified with isopropanol.
Again, the pattern of bands was uncommon and unexpected, hence we purified the mixture to remove residual enzyme and cut DNA with isopropanol. Analysing this on a gel that was run at 100V yielded a better result. The obtained bands met our expectations. For validation, a TLC was run with the samples, which again showed the slower migration of Val-Ind. Finally, we could submit our samples to the registry, as sequencing was positive for all linkers.
 "
"