 Home Home
 Team Team
 Project Project
 Parts Parts
 Protocol Protocol
 Notebook Notebook
 Results Results
 Safety Safety
 Human Practice Human Practice
|
Safety
- Before answering these questions on your team Safety page, be sure to read the Safety in iGEM page. and the FAQ section below.
Basic Safety Questions for iGEM 2013
- a. Please describe the chassis organism(s) you will be using for this project. If you will be using more than one chassis organism, provide information on each of them:

- *For additional organisms, please include a spreadsheet in your submission.
2. Highest Risk Group Listed:
1 ● Greater than 1 ○
- If you answered 1+, please also complete the iGEM Biosafety form part 2 for any organisms in this category.
3. List and describe all new or modified coding regions you will be using in your project.
(If you use parts from the 2013 iGEM Distribution without modifying them, you do not need to list those parts.)
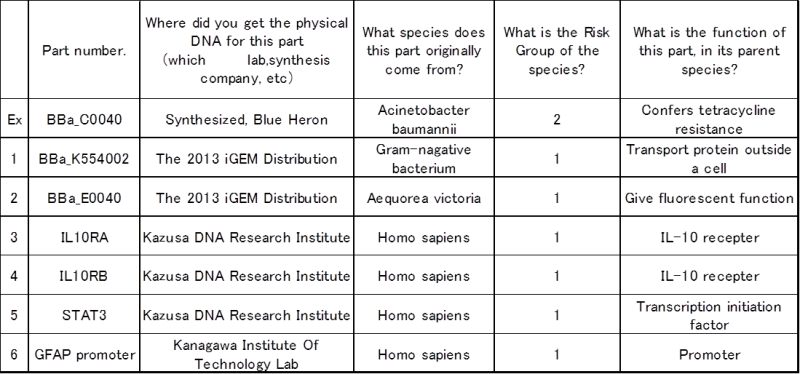
- *For additional coding regions, please include a spreadsheet in your submission.
4. Do the biological materials used in your lab work pose any of the following risks? Please describe.
- a. Risks to the safety and health of team members or others working in the lab?
We used E.coli Pro 5-alpha. It doesn’t cause irritation effect on the skin. And it doesn't sensitize us.
- b. Risks to the safety and health of the general public, if released by design or by accident?
We used E.coli Pro 5-alpha. It doesn’t cause irritation effect on the skin. And it doesn't sensitize us.
- c. Risks to the environment, if released by design or by accident?
Aquatic toxicity: Not harmful to the aquatic environment.
- d. Risks to security through malicious misuse by individuals, groups, or countries?
We discard the experimental dust after autoclave. Therefore there cannot be the thing leaking outside.
5. If your project moved from a small-scale lab study to become widely used as a commercial/industrial product, what new risks might arise?(Consider the different categories of risks that are listed in parts a-d of the previous question.)
Also, what risks might arise if the knowledge you generate or the methods you develop became widely available?
(Note: This is meant to be a somewhat open-ended discussion question.)
- It may fall into autoimmune disease by Th1 increasing excessively.
- α-hemolysin (Used as a tag to start it outside IL-12) provides hemolysis.
- When a human being took E coli, it is concerned whether you are safe.
6. Does your project include any design features to address safety risks?
(For example: kill switches, auxotrophic chassis, etc.)
Note that including such features is not mandatory to participate in iGEM, but many groups choose to include them.
Yes, our project includes it.
E.coli which we make by this project does not transcription if it does not receive IL-10.
7. What safety training has you received (or plans to receive in the future)? Provide a brief description, and a link to your institution’s safety training requirements, if available.
We took biosafety training by the class of the university.
8. Under what biosafety provisions will / do you work?
- a. Please provide a link to your institution biosafety guidelines.
http://kw.kait.jp/kw/top/index2.aspx
- b. Does your institution have an Institutional Biosafety Committee, or an equivalent group? If yes, have you discussed your project with them? Describe any concerns they raised with your project, and any changes you made to your project plan based on their review.
It is difficult to judge active having produced interleukin or not.
Therefore we did not judge active presence and decided to confirm whether IL-12 was produced.
- c. Does your country have national biosafety regulations or guidelines? If so, please provide a link to these regulations or guidelines if possible.
http://www.lifescience.mext.go.jp/files/pdf/n815_01.pdf
- d. According to the WHO Biosafety Manual, what is the Biosafety Level rating of your lab?(Check the summary table on page 3, and the fuller description that starts on page 9.)If your lab does not fit neatly into category 1, 2, 3, or 4, please describe its safety features [see 2013.igem.org/Safety for help].
We have a laboratory of biosafety level 1 and 2. Therefore we use them properly depending on a use.
|
 "
"





