Team:UChicago/Plan
From 2013.igem.org
Summer Plan and Challenges
Constructing the kerA BioBricks
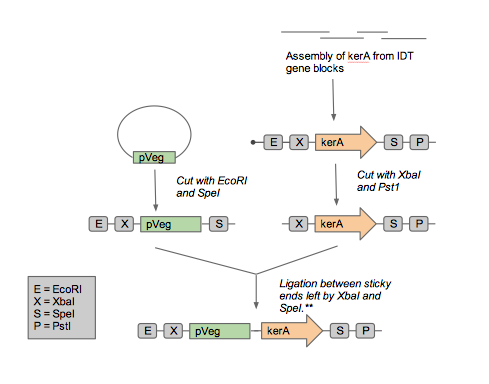
It was less costly to order gBlocks than to purchase the Bacillus licheniformis PWD-1 strain that has the kerA gene. Thus, we opted to assemble the kerA gene with gBlocks from IDT. Furthermore, the kerA sequence contains an EcoRI site and a PstI site, which would have to be removed for kerA to be used as a BioBrick, making the choice of getting gBlocks over the PWD-1 strain even more favorable. Once we carried out Gibson Assembly, we amplified the kerA sequence with PCR. We ran our PCR product in a gel and assumed that the right sequence was obtained based on the size of the DNA band. Once we had a lot of kerA DNA to work with, we decided it would be best to insert the BioBrick into an Amp resistant backbone provided in the iGEM kit considering our upstream parts are Cam resistant and the pUB110 B. subtilis plasmid is Kan resistant. To do so, we digested the PCR product and the backbone with EcoRI and PstI. We set up a ligation and transformed the plasmid into competent E. coli DH5-alpha. Unfortunately, we were unable to get any transformants. Prior to the transformation with the plasmid containing kerA, we tested the transformation efficiency of our competent cells so we discarded the possibility that we were performing the transformations incorrectly. Therefore, we thought that maybe the problem was with the assembly, PCR, digestion, or ligation of kerA. We tried using different conditions (temperatures, incubation time, and different enzymes) to eliminate our sources of error, but we were unable to get any transformants.
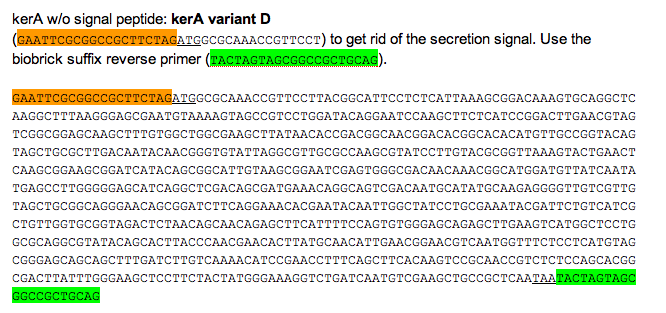
We would have carried on to alter the kerA BioBrick we first assembled so that it contained a his tag.
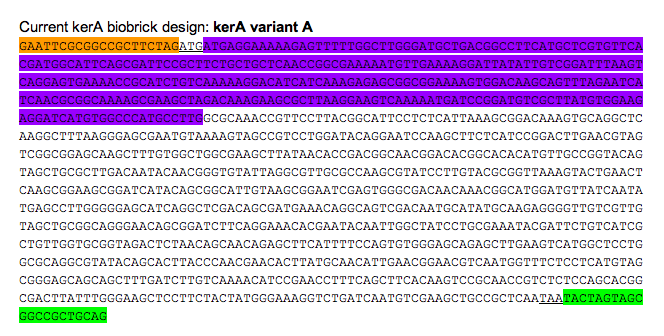
Orange: prefix
Green: suffix
Purple: kerA signal peptide
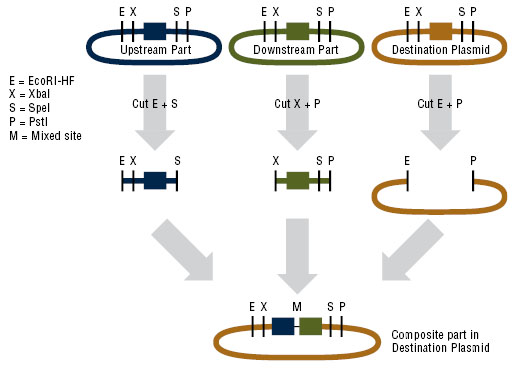
Constructing the pUB110 backbone
In order to express kerA in B. subtilis in high amounts, we need to use a high copy number plasmid. Since high copy number plasmids for B. subtilis are not available form the iGEM HQ, we decided to design our own. We derived our design from pUB110 because it has been widely used in B. subtilis.
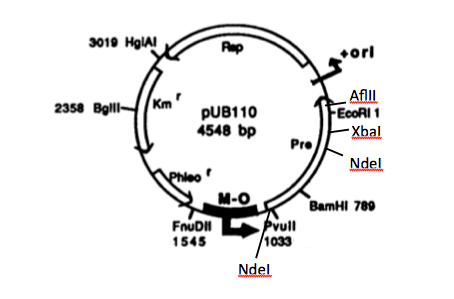
Source: Boe, L. A. R. S., Gros, M. F., te Riele, H. E. I. N., Ehrlich, S. D., & Gruss, A. (1989). Replication origins of single-stranded-DNA plasmid pUB110. Journal of bacteriology, 171(6), 3366-3372.
In the image, M-O represents the origin replication that is recognized by B. subtilis, while ori+ is the origin of replication used by S. aureus. Because this vector includes EcoRI and XbaI sites, we had to find a way to remove them before we turned the vector into a BioBrick backbone. We chose to digest pUB110 with NdeI and AflII, which would remove the Pre gene along with the EcoRI and XbaI sites from the vector. However, this gene is not essential for replication in B. subtilis. We then ran the digest in a gel and did a gel extraction to isolate the part of the vector that we needed.
In order to add in the BioBrick prefix and suffix to the vector, we designed a linker that was synthesized as two separate oligos. The DNA sequence of the two oligos are as follows:
5' attcgtcttaaggaattcgcggccgcttctagagtactagtagcggccgctgcagcatatgtcatac 3'
5' gtatgacatatgctgcagcggccgctactagtactctagaagcggccgcgaattccttaagacgaat 3'
We annealed the two oligos in duplex buffer (recipe from IDT) to use the duplex as a linker. Once the oligos were annealed, the linker was digested with NdeI and AflII. We then set up an overnight ligation of the digested, gel purified pUB110 and digested pUB110 linker, which would ideally lead to the formation of a backbone compatible with BioBricks. Nonetheless, when we were unable to get any transformants with our modified vector. To make sure that we weren't doing anything wrong with the transformation procedure of B. subtilis, we tried to transform the original pUB110. The transformation was successful, which led us to think that the problem may be with our modified vector. The failure of transformations could have been attributed to incomplete digestions, too little DNA retrieved from the gel extraction, or incomplete ligation. We attempted to address these potential problems by using new AflII and NdeI restriction enzymes with varying incubation times, different kits for gel extractions, and overnight ligations. Despite all our attempts, we did not successfully transformed B. subtilis with our modified backbone.
If our transformation of the modified pUB110 vector had worked, we would have picked transformants, grown them overnight to amplify the quantity of backbone DNA, and then done a miniprep to isolate the modified pUB110 backbone. We would have then sent the vector for sequencing. Once we verified that the backbone sequence were correct, we would have tested expression of the backbone. Using the NEB assembly kit, we would have digested an upstream part or promoter, a RFP BioBrick, and the modified pUB110 backbone. Once the construct were assembled, we would have transformed it into B. subtilis and checked for RFP expression.
Assembling plasmid for expression in B. subtilis
In order to express kerA in B. subtilis, we would have used the NEB assembly kit for the following parts:
Upstream part would be the constitutive promoter Pveg +RBS spoVG + SacB (BBa_K541501) found in a Cam resistant vector. Meanwhile, the downstream part would be the kerA variants without the signal peptide in the Amp resistant vector. And the modified pUB110 with Kan resistance would be the destination plasmid.
Source: New England Bio Labs
Note: In order to test expression of kerA with its native signal peptide, we would have instead used the constitutive promoter Pveg + RBS spoVG (BBa_K14305) as the upstream part.
Assembling plasmid for expression in E. coli BL21-De3
To test expression in E. coli, we would have used the constitutive expression cassette (BBa_K314100) as the upstream part, the kerA variants without the signal peptide and a high copy number vector with Kan resistance as a destination plasmid. If kerA expression in E. coli were toxic, we would have instead used the lac induced expression cassette (BBa_K314103) as the upstream part.
Testing KerA expression and activity in B. subtilis
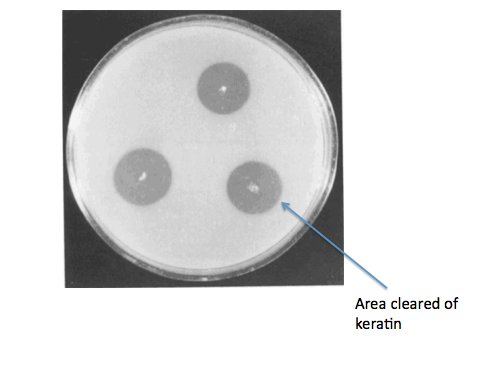
If everything had gone according to plan, we would have grown B. subtilis kerA transformants on keratin plates (made with LB agar + keratin from DMSO dissolved feathers) to determine secretion and activity of KerA. As a negative control for non-specific protease activity we would have instead used non-transformed B. subtilis. As a positive control, we would have also gotten B. licheniformis PWD-1 and grown it on the keratin plates. We would have used the clearing of keratin from the plate as a sign of kerA activity and secretion.
Source:
Wawrzkiewicz, K., Wolski, T., & Łobarzewski, J. (1991). Screening the keratinolytic activity of dermatophytes in vitro. Mycopathologia, 114(1), 1-8.
To be completely certain that kerA (his tagged) is being expressed in B. subtilis, we would have done a western blot on the cell lysis as well as on the liquid medium in which the transformants were grown. The western blot of the medium would indicate whether there was kerA secretion.
In order to have a more quantitative test of KerA activity, we would have used a keratin azure assay, which demonstrates keratinase activity based on a colorimetric reading.
Testing KerA expression and activity in E. coli BL21-De3
In E. coli transformants, we would have detected keratinase expression (his tagged) in the cell lysate by western blot. If expression of his tagged kerA is detected, we would have proceeded to do a keratin azure assay on the cell lysate.
Improve KerA activity by mutagenesis
If we had had more time, we would have used error prone PCR to introduce mutations in kerA as a way to isolate kerA variants with increased activity. We would have transformed the mutated kerA into B. subtilis and plated the bacteria on keratin plates. We would have then selected the mutants which demonstrated a greater clearing area around the B. subtilis colony. We would have followed with the study with a keratin azure assay to have more quantitative data on keratinase activity.
 "
"