Team:UGent/Project
From 2013.igem.org
| Line 6: | Line 6: | ||
{{:Team:UGent/Templates/ToggleBoxStart}} Read more about plasmids {{:Team:UGent/Templates/ToggleBoxStart1}}{{:Team:UGent/Templates/ToggleBoxStart2}} | {{:Team:UGent/Templates/ToggleBoxStart}} Read more about plasmids {{:Team:UGent/Templates/ToggleBoxStart1}}{{:Team:UGent/Templates/ToggleBoxStart2}} | ||
<html> | <html> | ||
| - | |||
<p>In industrial biotechnology, a common technique to express new synthetic products and pathways is the use of plasmids as vectors. Plasmids are easy to insert into cells and replicate independently from the genome, allowing strong gene expression. Overexpression is easily achieved by using plasmids with a medium or high copy number, different promoter systems, ribosome binding sites (RBS), etc. Thanks to plasmids, the industrial biotechnology has grown substantially over the past years. However, the use of plasmids entails some important disadvantages.</p> | <p>In industrial biotechnology, a common technique to express new synthetic products and pathways is the use of plasmids as vectors. Plasmids are easy to insert into cells and replicate independently from the genome, allowing strong gene expression. Overexpression is easily achieved by using plasmids with a medium or high copy number, different promoter systems, ribosome binding sites (RBS), etc. Thanks to plasmids, the industrial biotechnology has grown substantially over the past years. However, the use of plasmids entails some important disadvantages.</p> | ||
| - | |||
</html> | </html> | ||
{{:Team:UGent/Templates/ToggleBoxEnd}} | {{:Team:UGent/Templates/ToggleBoxEnd}} | ||
<html> | <html> | ||
| - | |||
<p>Therefore a new method was developed for the overexpression of a gene of interest in the bacterial chromosome: Chemically Inducible Chromosomal evolution (CIChE). In this technique the chromosome is evolved to contain a higher number of gene copies by adding a chemical inducer. The original model for CIChE, however, results in bacterial strains containing a large number of antibiotic resistance genes.</p> | <p>Therefore a new method was developed for the overexpression of a gene of interest in the bacterial chromosome: Chemically Inducible Chromosomal evolution (CIChE). In this technique the chromosome is evolved to contain a higher number of gene copies by adding a chemical inducer. The original model for CIChE, however, results in bacterial strains containing a large number of antibiotic resistance genes.</p> | ||
| - | |||
</html> | </html> | ||
{{:Team:UGent/Templates/ToggleBoxStart}} Read more about CIChE{{:Team:UGent/Templates/ToggleBoxStart1}}{{:Team:UGent/Templates/ToggleBoxStart2}} | {{:Team:UGent/Templates/ToggleBoxStart}} Read more about CIChE{{:Team:UGent/Templates/ToggleBoxStart1}}{{:Team:UGent/Templates/ToggleBoxStart2}} | ||
<html> | <html> | ||
| - | |||
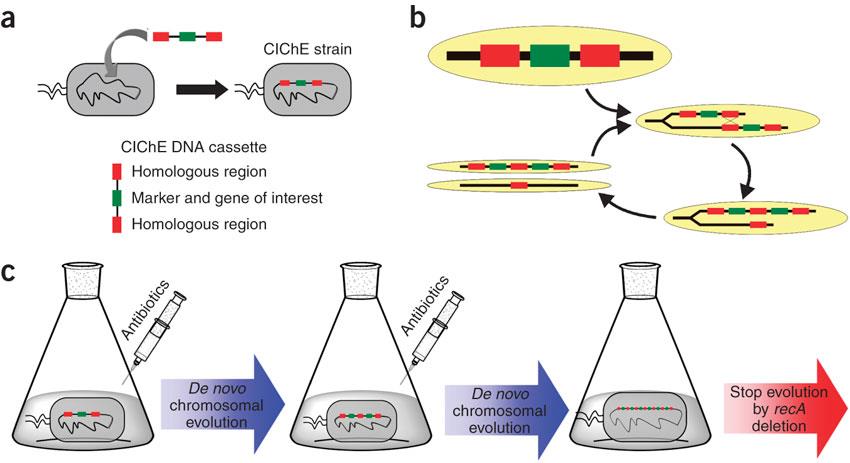
<p>In 2009, Tyo et al. developed a technique for the stable, high copy expression of a gene of interest in E. coli without the use of high copy number plasmids, thus avoiding their previously stated negative characteristics. They called this plasmid-free, high gene copy expression system ‘chemically inducible chromosomal evolution’. In this method, the gene of interest is integrated in the microbial genome and then amplified to achieve multiple copies and reach the desired expression level. Genomic integration guarantees ordered inheritance, resolving the problem of allele segregation. | <p>In 2009, Tyo et al. developed a technique for the stable, high copy expression of a gene of interest in E. coli without the use of high copy number plasmids, thus avoiding their previously stated negative characteristics. They called this plasmid-free, high gene copy expression system ‘chemically inducible chromosomal evolution’. In this method, the gene of interest is integrated in the microbial genome and then amplified to achieve multiple copies and reach the desired expression level. Genomic integration guarantees ordered inheritance, resolving the problem of allele segregation. | ||
CIChE works as follows: First a construct, containing the gene(s) of interest and the antibiotic marker chloramphenicol acetyl transferase (<i>cat</i>) flanked by homologous regions, is delivered to and subsequently integrated into the ''E. coli'' genome. The construct can be amplified in the genome through tandem gene duplication by recA homologous recombination. Then the strain is cultured in increasing concentrations of chloramphenicol, providing a growth advantage for cells with increased repeats of the construct and thereby selecting for bacteria with a higher gene copy number (<b>Figure</b>).</p> | CIChE works as follows: First a construct, containing the gene(s) of interest and the antibiotic marker chloramphenicol acetyl transferase (<i>cat</i>) flanked by homologous regions, is delivered to and subsequently integrated into the ''E. coli'' genome. The construct can be amplified in the genome through tandem gene duplication by recA homologous recombination. Then the strain is cultured in increasing concentrations of chloramphenicol, providing a growth advantage for cells with increased repeats of the construct and thereby selecting for bacteria with a higher gene copy number (<b>Figure</b>).</p> | ||
| - | |||
</html> | </html> | ||
[[File:UGent_2013_CIChE.jpg|thumb|400px|center|Tyo et al., 2009]] | [[File:UGent_2013_CIChE.jpg|thumb|400px|center|Tyo et al., 2009]] | ||
<html> | <html> | ||
| - | |||
<p>This process is called chromosomal evolution. When the desired gene copy number is reached, <i>recA</i> is deleted, thereby fixing the copy number.</p> | <p>This process is called chromosomal evolution. When the desired gene copy number is reached, <i>recA</i> is deleted, thereby fixing the copy number.</p> | ||
| - | |||
</html> | </html> | ||
{{:Team:UGent/Templates/ToggleBoxEnd}} | {{:Team:UGent/Templates/ToggleBoxEnd}} | ||
<html> | <html> | ||
| - | |||
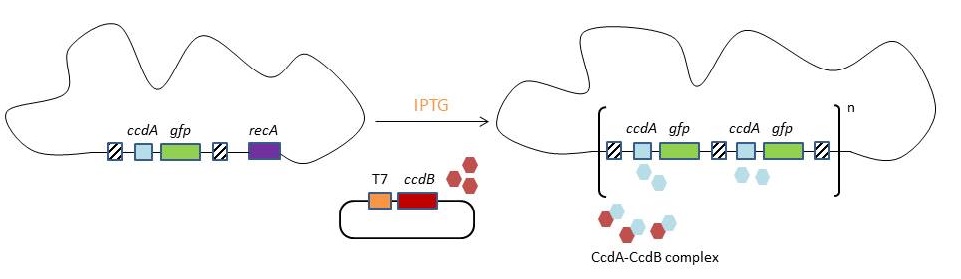
<p>To make this valuable technique more widely applicable in the industry, we developed a model for chromosomal evolution based on a toxin-antitoxin system instead of antibiotic resistance.</p> | <p>To make this valuable technique more widely applicable in the industry, we developed a model for chromosomal evolution based on a toxin-antitoxin system instead of antibiotic resistance.</p> | ||
</body> | </body> | ||
| Line 37: | Line 28: | ||
{{:Team:UGent/Templates/ToggleBoxStart}} Read more about antibiotic resistance{{:Team:UGent/Templates/ToggleBoxStart1}}{{:Team:UGent/Templates/ToggleBoxStart2}} | {{:Team:UGent/Templates/ToggleBoxStart}} Read more about antibiotic resistance{{:Team:UGent/Templates/ToggleBoxStart1}}{{:Team:UGent/Templates/ToggleBoxStart2}} | ||
<html> | <html> | ||
| - | |||
<p>Antibiotic resistance is the resistance of a bacterium to an antibacterial medicine a.k.a. antibiotic to which it was originally sensitive. When a pathogenic bacterium has acquired resistance to a certain antibiotic, infections of this bacterium can no longer be treated with the antibiotic in question since the bacterium has become insensitive to the antibiotic. | <p>Antibiotic resistance is the resistance of a bacterium to an antibacterial medicine a.k.a. antibiotic to which it was originally sensitive. When a pathogenic bacterium has acquired resistance to a certain antibiotic, infections of this bacterium can no longer be treated with the antibiotic in question since the bacterium has become insensitive to the antibiotic. | ||
Overuse and misuse of antibiotics has accelerated the emergence and spread of resistant bacteria. Today antibiotic resistance has become a fairly well-known term as a result of the recurring appearance of multi-drug resistant bacteria such as MRSA and VRE in media, awareness campaigns etc. These bacteria have become resistant to the commonly used antibiotics. As a result infections are very difficult to treat. The emergence of these hard-to-kill pathogenic bacteria poses a serious risk to public health. | Overuse and misuse of antibiotics has accelerated the emergence and spread of resistant bacteria. Today antibiotic resistance has become a fairly well-known term as a result of the recurring appearance of multi-drug resistant bacteria such as MRSA and VRE in media, awareness campaigns etc. These bacteria have become resistant to the commonly used antibiotics. As a result infections are very difficult to treat. The emergence of these hard-to-kill pathogenic bacteria poses a serious risk to public health. | ||
<BR><BR> | <BR><BR> | ||
In biotechnology antibiotic resistance genes (the genes that make a bacterium resistant to a certain antibiotic) are often used as selectable markers. The best known application is probably the selection of plasmids. In the original CIChE technique a chloramphenicol resistance gene is used as selectable marker. During chromosomal evolution the antibiotic resistance gene is duplicated since it is the driving pressure for the tandem duplication of the CIChE-construct. As a result the final bacterial strain carries multiple antibiotic resistance genes. The creation of such a strain raises several safety concerns as bacteria can pass resistance genes on to other, possibly pathogenic, bacteria in the environment through a process called horizontal gene transfer. Although this is very unlikely to occur the possibility of horizontal gene transfer cannot be entirely ruled out. We will try to replace the use of antibiotic resistance genes with a toxin-antitoxin system. By eliminating the need for antibiotic resistance genes, CIChE could be applied in the industry without having to worry about the possibility of horizontal gene transfer and the spread of antibiotic resistance.</p> | In biotechnology antibiotic resistance genes (the genes that make a bacterium resistant to a certain antibiotic) are often used as selectable markers. The best known application is probably the selection of plasmids. In the original CIChE technique a chloramphenicol resistance gene is used as selectable marker. During chromosomal evolution the antibiotic resistance gene is duplicated since it is the driving pressure for the tandem duplication of the CIChE-construct. As a result the final bacterial strain carries multiple antibiotic resistance genes. The creation of such a strain raises several safety concerns as bacteria can pass resistance genes on to other, possibly pathogenic, bacteria in the environment through a process called horizontal gene transfer. Although this is very unlikely to occur the possibility of horizontal gene transfer cannot be entirely ruled out. We will try to replace the use of antibiotic resistance genes with a toxin-antitoxin system. By eliminating the need for antibiotic resistance genes, CIChE could be applied in the industry without having to worry about the possibility of horizontal gene transfer and the spread of antibiotic resistance.</p> | ||
| - | |||
</html> | </html> | ||
| Line 48: | Line 37: | ||
<html> | <html> | ||
| - | |||
<h1>Our model</h1> | <h1>Our model</h1> | ||
| Line 55: | Line 43: | ||
<p>In our opinion it is possible to eliminate the need for antibiotics by using a toxin-antitoxin system. These systems are widely distributed among bacteria and archaea. The toxins produced by these systems can slow down or stop cell growth by interfering with certain molecules that are essential in cellular processes like DNA replication, cell wall synthesis, ATP synthesis etc.. Under normal conditions, the toxin is inhibited by the antitoxin, which is encoded in the same operon as the toxin. Research revealed different types of TA systems.</p> | <p>In our opinion it is possible to eliminate the need for antibiotics by using a toxin-antitoxin system. These systems are widely distributed among bacteria and archaea. The toxins produced by these systems can slow down or stop cell growth by interfering with certain molecules that are essential in cellular processes like DNA replication, cell wall synthesis, ATP synthesis etc.. Under normal conditions, the toxin is inhibited by the antitoxin, which is encoded in the same operon as the toxin. Research revealed different types of TA systems.</p> | ||
| - | |||
</html> | </html> | ||
{{:Team:UGent/Templates/ToggleBoxStart}} Read more about the different types of TA systems {{:Team:UGent/Templates/ToggleBoxStart1}}{{:Team:UGent/Templates/ToggleBoxStart2}} | {{:Team:UGent/Templates/ToggleBoxStart}} Read more about the different types of TA systems {{:Team:UGent/Templates/ToggleBoxStart1}}{{:Team:UGent/Templates/ToggleBoxStart2}} | ||
<html> | <html> | ||
| - | |||
<p> | <p> | ||
<b>Type I</b> | <b>Type I</b> | ||
| Line 73: | Line 59: | ||
In the last category of TA systems, an RNA antitoxin directly inhibits the toxin. These RNA antitoxins are pseudoknots, which contain internal stemloops. The toxin and its antidote are once more encoded by the same operon. A transcriptional terminator between the two genes, regulates the ratio of toxin and antitoxin in the cell.</p> | In the last category of TA systems, an RNA antitoxin directly inhibits the toxin. These RNA antitoxins are pseudoknots, which contain internal stemloops. The toxin and its antidote are once more encoded by the same operon. A transcriptional terminator between the two genes, regulates the ratio of toxin and antitoxin in the cell.</p> | ||
| - | |||
</html> | </html> | ||
{{:Team:UGent/Templates/ToggleBoxEnd}} | {{:Team:UGent/Templates/ToggleBoxEnd}} | ||
<html> | <html> | ||
| - | |||
<h3> CcdA/CcdB </h3> | <h3> CcdA/CcdB </h3> | ||
<p>This type II TA system is the first identified and best studied of all TA systems, which is why we have chosen to use it in our model. The target of the CcdB protein is the A subunit of DNA gyrase. This gyrase is an essential type II topoisomerase, occurring in all bacteria but not in eukaryotes. Bacterial gyrases have the property to introduce negative supercoils into DNA, which makes them unique among topoisomerases. | <p>This type II TA system is the first identified and best studied of all TA systems, which is why we have chosen to use it in our model. The target of the CcdB protein is the A subunit of DNA gyrase. This gyrase is an essential type II topoisomerase, occurring in all bacteria but not in eukaryotes. Bacterial gyrases have the property to introduce negative supercoils into DNA, which makes them unique among topoisomerases. | ||
| Line 84: | Line 68: | ||
<h2>Construct</h2> | <h2>Construct</h2> | ||
| - | |||
</html> | </html> | ||
[[File:UGent_2013_Model.jpg|thumb|700px|center|Our model for CIChE]] | [[File:UGent_2013_Model.jpg|thumb|700px|center|Our model for CIChE]] | ||
{{:Team:UGent/Templates/Footer}} | {{:Team:UGent/Templates/Footer}} | ||
{{:Team:UGent/Templates/BaseSponsor}} | {{:Team:UGent/Templates/BaseSponsor}} | ||
 "
"