Team:uOttawa/project
From 2013.igem.org

Background
The Type-I Incoherent Feedforward Loop and Fold-Change Detection
The type-I incoherent feedforward loop (I1-FFL) is a gene network in which protein X activates a gene Z while simultaneously activating the production of a repressor of gene Z, labeled Y.

For this year’s project, we are aiming to construct an I1-FFL that can detect fold-changes in the levels of protein X. In other words, the expression of Z would be reliant on the relative change in X as opposed to absolute values of X. For example, if X changes from an arbitrary concentration of 1 to an arbitrary concentration of 5, the expression from promoter Z would be exactly the same as that resulting from a change from a concentration of 5 to a concentration of 25 of X (a fold-change by a factor of 5 in both cases).
How Fold-Change Detection is Achieved
This fold-change detection is enabled through the repressor Y. Take the case when activator X, repressor Y, and protein Z each have an arbitrary concentration of 1 in the cell (1:1:1 ratio). If the concentration of X is doubled, the ratio between X and Y now becomes 2:1. X immediately activates Z, but since the repressor Y takes time to fold, it lags behind, and the concentration of Z spikes to 2 due to the activation. When Y folds into its active form and carries out its repressive activity, the concentration of Z returns to its initial concentration of 1. This is because at this point, X and Y return to a 1:1 ratio in the cell. In order for the concentration of Z to reach 2 once again, the X:Y ratio must first return to 2:1 - thus, X has to reach a concentration of 4 before the same output of Z is produced. In this way, fold-change detection is achieved.

Engineering our System
The Design of our Gene Network and the Interactions Between Components
In our proposed design, rtTA will act as our activator (X), Lacl as our repressor (Y), and sfGFP as our reporter protein (Z). Each part is detectable via a different fluorescent protein. A visual representation of our pathway is shown in Figure 3.
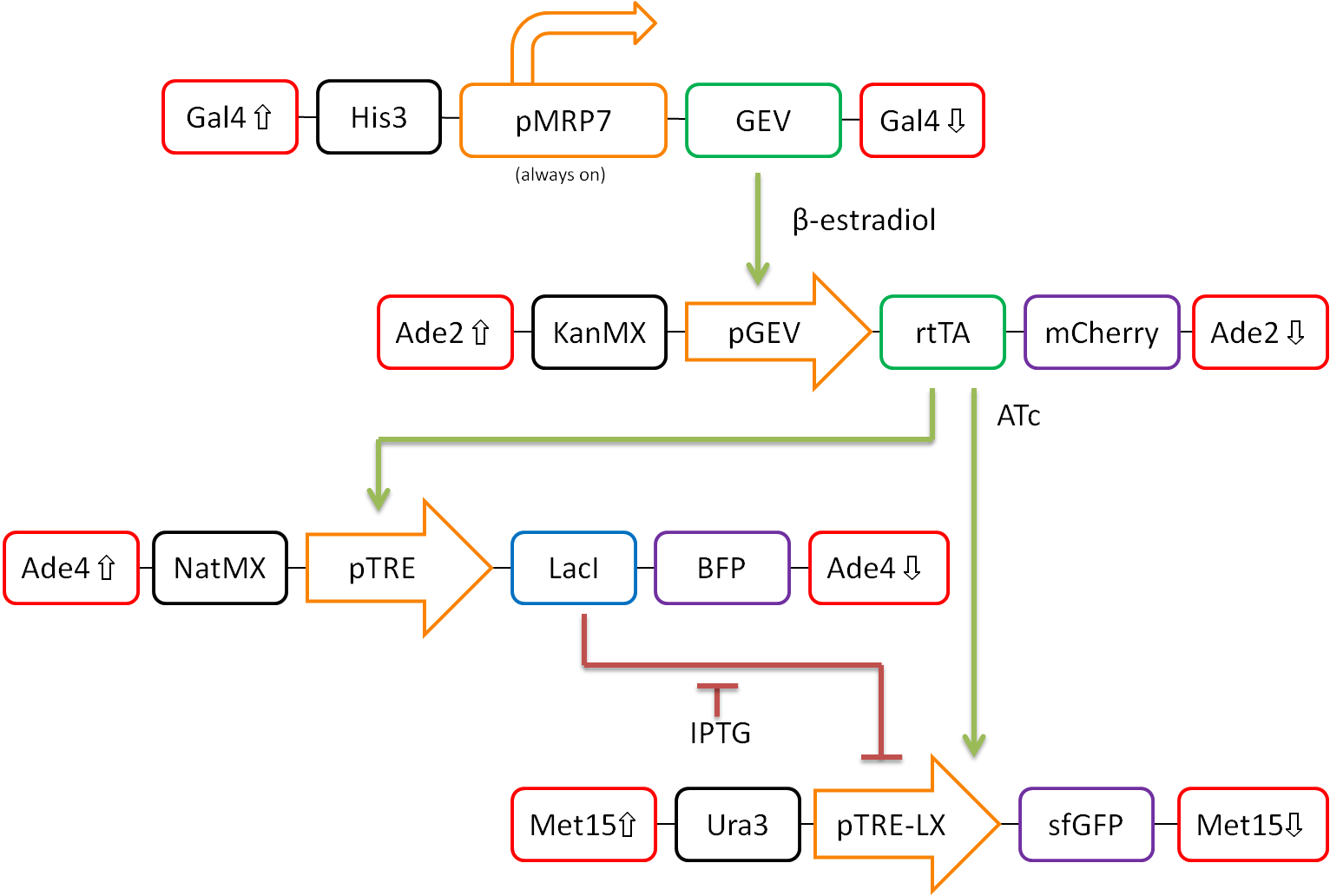
This network design allows for calibration of the network via tunable levels of β-estradiol, allowing for accurate control of the amount of rtTA(X) in the cell. This will allow us to ensure that fold-change detection works as theorized in our network.
Furthermore, activation by rtTA is mediated by varying levels of anhydrotetracycline (ATc), and LacI repression is mediated by varying levels of Isopropyl β-D-1-thiogalactopyranoside (IPTG). These methods of mediation allow for high tunability of the system, and we should be able to find a concentration range of ATc and IPTG at which the system will work as desired.
The system will then be characterized extensively. The fluorescent markers tagged to each component of the network will allow for simple temporal analysis using flow cytometry. This will allow for a high-throughput analysis of the activity of our network.
Potential Applications
A Fold-Change Detection System for Toxic Molecules
Once our system is tuned and is working, it can be modified to act as a fold-change detector for toxic molecules. By replacing the pGEV promoter in front of the rtTA gene with a promoter that is inducible by a toxic molecule, the amount of rtTA produced will be directly correlated to the concentration of the toxic molecule in the cellular environment.

This detector system is advantageous in that it detects fold-changes rather than changes in absolute values, which gives meaning to a signal in reference to the background signal level. This allows for the network to respond only to a signal that rises significantly above the background noise.
We have showed....
- This
- That
- And this some more
pGal1
This is a blurb. A what? A blurb. Oh, a blurb. This is a blurb. A what? A what? A blurb. A blurb. Oh, a blurb. This is a blurb. A what? A what? A what? A blurb. A blurb. A blurb. Oh, a blurb. This is a blurb.
This is a blurb. A what? A blurb. Oh, a blurb. This is a blurb. A what? A what? A blurb. A blurb. Oh, a blurb. This is a blurb. A what? A what? A what? A blurb. A blurb. A blurb. Oh, a blurb. This is a blurb.
This is a blurb. A what? A blurb. Oh, a blurb. This is a blurb. A what? A what? A blurb. A blurb. Oh, a blurb. This is a blurb. A what? A what? A what? A blurb. A blurb. A blurb. Oh, a blurb. This is a blurb.
pLX
This is a blurb. A what? A blurb. Oh, a blurb. This is a blurb. A what? A what? A blurb. A blurb. Oh, a blurb. This is a blurb. A what? A what? A what? A blurb. A blurb. A blurb. Oh, a blurb. This is a blurb.
This is a blurb. A what? A blurb. Oh, a blurb. This is a blurb. A what? A what? A blurb. A blurb. Oh, a blurb. This is a blurb. A what? A what? A what? A blurb. A blurb. A blurb. Oh, a blurb. This is a blurb.
This is a blurb. A what? A blurb. Oh, a blurb. This is a blurb. A what? A what? A blurb. A blurb. Oh, a blurb. This is a blurb. A what? A what? A what? A blurb. A blurb. A blurb. Oh, a blurb. This is a blurb.
TX
This is a blurb. A what? A blurb. Oh, a blurb. This is a blurb. A what? A what? A blurb. A blurb. Oh, a blurb. This is a blurb. A what? A what? A what? A blurb. A blurb. A blurb. Oh, a blurb. This is a blurb.
This is a blurb. A what? A blurb. Oh, a blurb. This is a blurb. A what? A what? A blurb. A blurb. Oh, a blurb. This is a blurb. A what? A what? A what? A blurb. A blurb. A blurb. Oh, a blurb. This is a blurb.
This is a blurb. A what? A blurb. Oh, a blurb. This is a blurb. A what? A what? A blurb. A blurb. Oh, a blurb. This is a blurb. A what? A what? A what? A blurb. A blurb. A blurb. Oh, a blurb. This is a blurb.
Collaboration: Waterloo
This year, we had the opportunity to collaborate with the iGEM team from the University of Waterloo. We were asked to aid them in the construction of four large DNA constructs for their project, as illustrated below:

In order to do so, the uOttawa iGEM Team designed a total of 36 primers for the uWaterloo team. Because many of the individual parts were very short, an experimental oligonucleotide-based synthesis method was used, in combination with a modified overlap extension PCR assembly method. A very detailed protocol was written for the uWaterloo team, and they were successfully able to construct their DNA constructs using our method.
In exchange for the construction aid, the uWaterloo team compiled a list of differential equations that were used to model our synthetic biological system. The equations were also implemented in MATLAB for us by the uWaterloo team. Thanks for the incredible help, Paul and the uWaterloo team! Learn more.
Collaboration: Purdue
In addition to our wet lab collaboration with Waterloo, we were also able to participate in the largest collaborative project in the history of iGEM, in partnership with Purdue University. Purdue contacted many iGEM teams across all corners of the world, and together we worked to propose a new characterization standard for the Registry of Standard Biological Parts. Thanks for the invitation, Purdue! Learn more.

References
- 1. Daniel R, Rubens JR, Sarpeshkar R, Lu TK (2013) Synthetic analog computation in living cells. Nature 497: 619–623
- 2. Ellis T, Wang X, Collins JJ. Diversity-based, model-guided construction of synthetic gene networks with predicted functions. Nat Biotechnol. 2009;27:465
- 3. Gibson D. G., Young L., Chuang R. Y., Venter J. C., Hutchison C. A., 3rd., Smith H. O. (2009). Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat. Methods 6, 343–345. doi: 10.1038/nmeth.1318.
- 4. Goentoro L, Shoval O, Kirschner MW, Alon U (2009) The incoherent feedforward loop can provide fold-change detection in gene regulation. Molecular Cell 36: 894–899.
- 5. Ho SN, Hunt HD, Horton RM, Pullen JK, Pease LR (1989) Site-Directed Mutagenesis by Overlap Extension Using the Polymerase Chain-Reaction. Gene 77: 51–59.
- 6. Jedrysiak, Daniel K. "Construction and Characterization of Gene Regulatory Networks in Yeast." Order No. MR86054 University of Ottawa (Canada), 2013. Ann Arbor: ProQuest. Web. 27 Sep. 2013.
- 7. Khmelinskii, A., P.J. Keller, A. Bartosik, M. Meurer, J.D. Barry, B.R. Mardin, A. Kaufmann, S. Trautmann, M. Wachsmuth, G. Pereira, W. Huber, E. Schiebel and M. Knop (2012). Tandem fluorescent protein timers for in vivo analysis of protein dynamics. Nat. Biotechnol., 30:708-714.
- 8. Kuttykrishnan S, Sabina J, Langton LL, Johnston M, Brent MR. A quantitative model of glucose signaling in yeast reveals an incoherent feed forward loop leading to a specific, transient pulse of transcription. Proc Natl Acad Sci USA. 2010;107:16743–16748. doi: 10.1073/pnas.0912483107.
- 9. Mads Kærn & Ron Weiss. Synthetic gene regulatory systems. In: Systems Modeling in Cellular Biology. Z. Sallasi et al. (Eds.). MIT Press (2006).
- 10. Mangan S, Alon U. Structure and function of the feed-forward loop network motif. Proc Natl Acad Sci USA. 2003;100:11980–85. Pioneering study of the functional significance of network motifs.
- 11. McIsaac RS, et al. Fast-acting and nearly gratuitous induction of gene expression and protein depletion in Saccharomyces cerevisiae. Molecular biology of the cell. 2011;22:4447–4459.
- 12. Nevozhay D, Adams RM, Murphy KF, Josic K, Balázsi G. Negative autoregulation linearizes the dose-response and suppresses the heterogeneity of gene expression. Proc Natl Acad Sci USA. 2009;106(13):5123–5128.
 "
"

