Template:Kyoto/Notebook/Sep 26
From 2013.igem.org
(Difference between revisions)
(→Electrophoresis) |
(→Colony PCR) |
||
| (40 intermediate revisions not shown) | |||
| Line 2: | Line 2: | ||
===Electrophoresis=== | ===Electrophoresis=== | ||
<div class="experiment"> | <div class="experiment"> | ||
| - | <span class="author"> | + | <span class="author">No name</span> |
{| class="wikitable" | {| class="wikitable" | ||
!Lane||Sample||Enzyme1||Enzyme2 | !Lane||Sample||Enzyme1||Enzyme2 | ||
|- | |- | ||
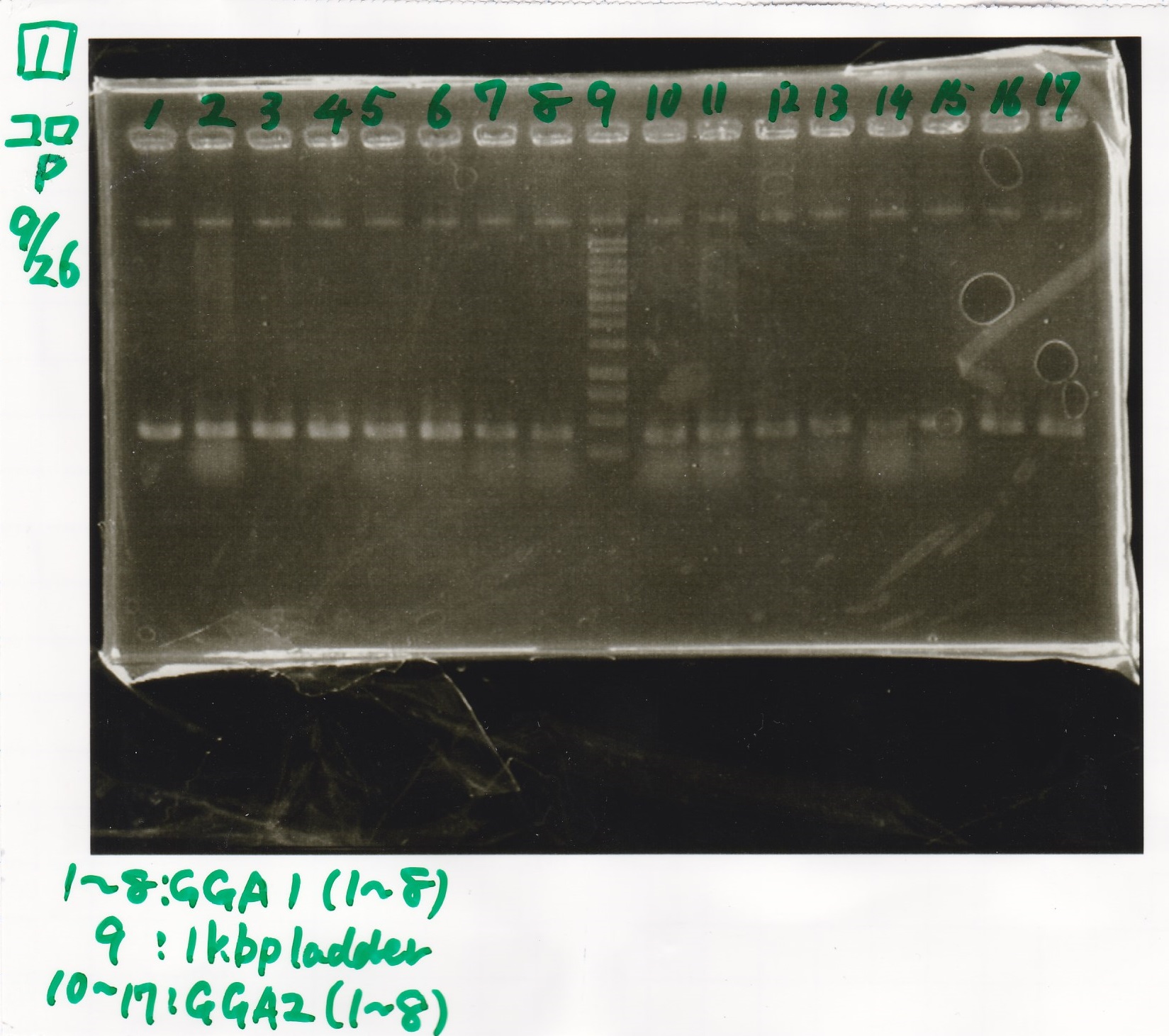
| - | |1||GGA1-1|| || | + | |1||GGA1-1||--||-- |
|- | |- | ||
| - | |2||GGA1-2|| || | + | |2||GGA1-2||--||-- |
|- | |- | ||
| - | |3||GGA1-3|| || | + | |3||GGA1-3||--||-- |
|- | |- | ||
| - | |4||GGA1-4|| || | + | |4||GGA1-4||--||-- |
|- | |- | ||
| - | |5||GGA1-5|| || | + | |5||GGA1-5||--||-- |
|- | |- | ||
| - | |6||GGA1-6|| || | + | |6||GGA1-6||--||-- |
|- | |- | ||
| - | |7||GGA1-7|| || | + | |7||GGA1-7||--||-- |
|- | |- | ||
| - | |8||GGA1-8|| || | + | |8||GGA1-8||--||-- |
|- | |- | ||
| - | |9||1kbp ladder|| || | + | |9||1kbp ladder||--||-- |
|- | |- | ||
| - | |10||GGA2-1|| || | + | |10||GGA2-1||--||-- |
|- | |- | ||
| - | |11||GGA2-2|| || | + | |11||GGA2-2||--||-- |
|- | |- | ||
| - | |12||GGA2-3|| || | + | |12||GGA2-3||--||-- |
|- | |- | ||
| - | |13||GGA2-4|| || | + | |13||GGA2-4||--||-- |
|- | |- | ||
| - | |14||GGA2-5|| || | + | |14||GGA2-5||--||-- |
|- | |- | ||
| - | |15||GGA2-6|| || | + | |15||GGA2-6||--||-- |
|- | |- | ||
| - | |16||GGA2-7|| || | + | |16||GGA2-7||--||-- |
|- | |- | ||
| - | |17||GGA2-8|| || | + | |17||GGA2-8||--||-- |
|} | |} | ||
| - | [[File: | + | [[File:Igku_0926_E1.jpg]]<br> |
| - | + | ||
{| class="wikitable" | {| class="wikitable" | ||
!Lane||Sample||Enzyme1||Enzyme2 | !Lane||Sample||Enzyme1||Enzyme2 | ||
|- | |- | ||
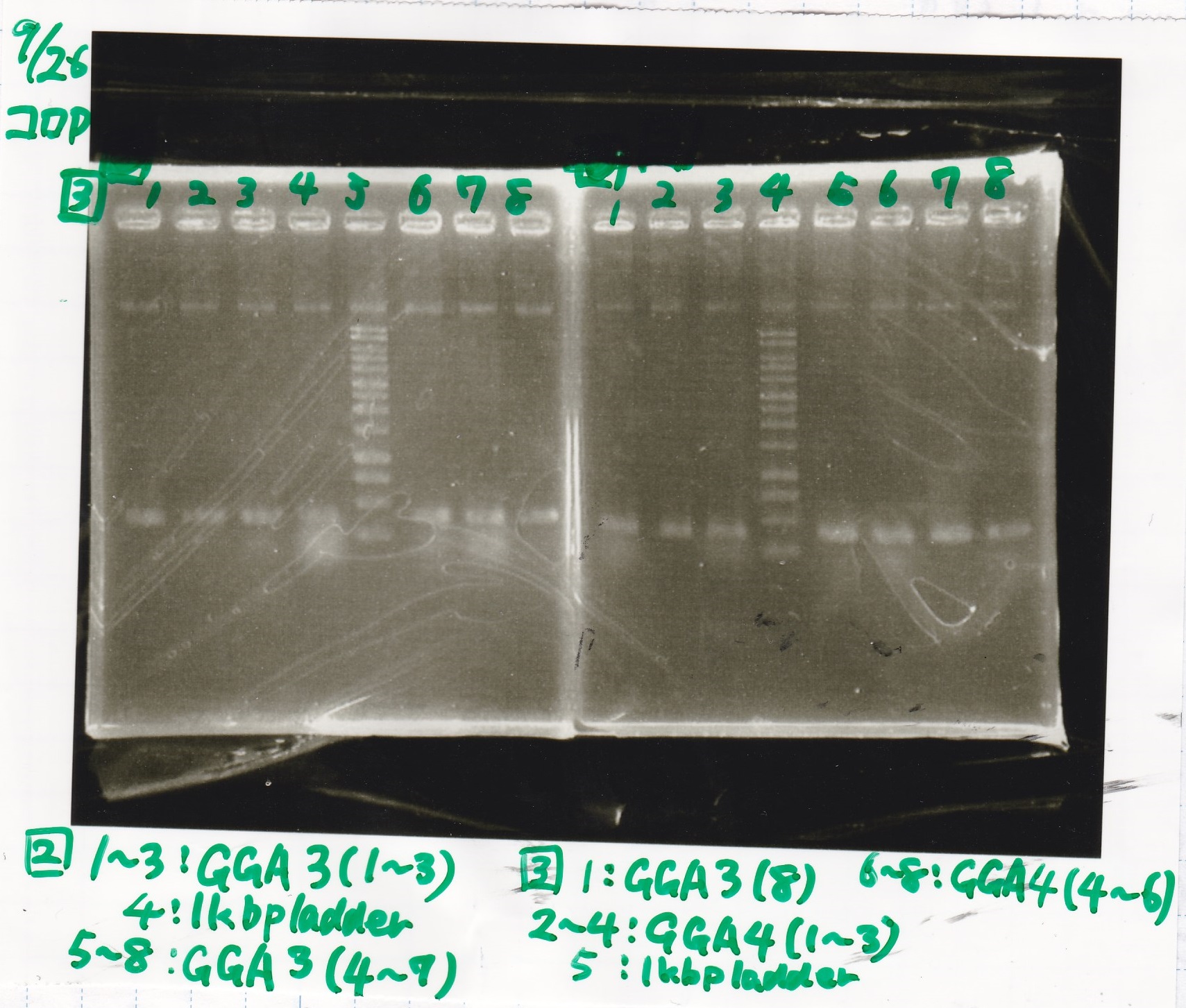
| - | |1||GGA3-1|| || | + | |1||GGA3-1||--||-- |
|- | |- | ||
| - | |2||GGA3-2|| || | + | |2||GGA3-2||--||-- |
|- | |- | ||
| - | |3||GGA3-3|| || | + | |3||GGA3-3||--||-- |
|- | |- | ||
| - | |4||1kbp ladder|| || | + | |4||1kbp ladder||--||-- |
|- | |- | ||
| - | |5||GGA3-4|| || | + | |5||GGA3-4||--||-- |
|- | |- | ||
| - | |6||GGA3-5|| || | + | |6||GGA3-5||--||-- |
|- | |- | ||
| - | |7||GGA3-6|| || | + | |7||GGA3-6||--||-- |
|- | |- | ||
| - | |8||GGA3-7|| || | + | |8||GGA3-7||--||-- |
|} | |} | ||
| - | [[File: | + | |
| + | {| class="wikitable" | ||
| + | !Lane||Sample||Enzyme1||Enzyme2 | ||
| + | |- | ||
| + | |1||GGA3-8||--||-- | ||
| + | |- | ||
| + | |2||GGA4-1||--||-- | ||
| + | |- | ||
| + | |3||GGA4-2||--||-- | ||
| + | |- | ||
| + | |4||GGA4-3||--||-- | ||
| + | |- | ||
| + | |5||1kbp ladder||--||-- | ||
| + | |- | ||
| + | |6||GGA4-4||--||-- | ||
| + | |- | ||
| + | |7||GGA4-5||--||-- | ||
| + | |- | ||
| + | |8||GGA4-6||--||-- | ||
| + | |} | ||
| + | [[File:Igku_0926_E2.jpg]]<br> | ||
| + | |||
| + | |||
| + | {| class="wikitable" | ||
| + | !Lane||Sample||Enzyme1||Enzyme2 | ||
| + | |- | ||
| + | |1||GGA4-7||--||-- | ||
| + | |- | ||
| + | |2||GGA4-8||--||-- | ||
| + | |- | ||
| + | |3||1kbp ladder||--||-- | ||
| + | |- | ||
| + | |4||GGA7-1||--||-- | ||
| + | |- | ||
| + | |5||GGA16-1||--||-- | ||
| + | |- | ||
| + | |6||NC(RBS-GFP-DT)||--||-- | ||
| + | |} | ||
| + | [[File:Igku_0926_E3.jpg]]<br> | ||
</div> | </div> | ||
| + | ===Ligasion(Golden Gate Assenbly)=== | ||
| + | <div class="experiment"> | ||
| + | <span class="author">No name</span> | ||
| + | {| class="wikitable" | ||
| + | !state||colspan="2"|Vector||colspan="2"|Inserter1||colspan="2"|Inserter2||colspan="2"|Inserter3||colspan="2"|Inserter4||colspan="2"|Inserter5||(NEB)T4 ligase||(NEB)10*T4 ligase||MilliQ | ||
| + | |- | ||
| + | |experiment||RBS-GFP-DT||1.0 µL||Pcon-pT181 antisense(E-2A)||0.6µL||DT(2-1)||0.5µL||Pcon-pT181 attenuator(1-SA)||2.1 µL||--||--||--||--||0.5 µL||0.5 µL||up to 5 µL | ||
| + | |- | ||
| + | |experiment||pSB1C3(EcoRI&SpeI)||1.8 µL||Pcon-pT181 antisense(E-2A)||0.9 µL||DT(2-1)||0.5 µL||Pcon-GFP-DT(1-SA)||1.5 µL||--||--||--||--||0.5 µL||0.5 µL||up to 5 µL | ||
| + | |- | ||
| + | |experiment||RBS-GFP-DT||1.0 µL||Ptet(E-0)||0.7µL||Pcon pT181 attenuator(0-1A)||0.3µL||tet aptamer(1-3)||0.3 µL||Pcon-tetR-DT(3-2A)||1.1 µL||Ptet(2-5)||0.3 µL||0.5 µL||0.5 µL||up to 5 µL | ||
| + | |- | ||
| + | |experiment||RBS-GFP-DT||1.0 µL||Ptet(E-0)||0.7 µL||Pcon-pT181 attenuator-DT(0-1A)||1.0 µL||Pcon-tetR-DT(1-2A)||1.0 µL||Ptet(2-5)||0.3µL||--||--||0.5 µL||0.5 µL||up to 5 µL | ||
| + | |- | ||
| + | |experiment||RBS-GFP-DT||1.0 µL||Pcon-pT181 attenuator-tetR-DT(E-1A)||1.0 µL||Pcon antisense-spinach-DT(1-2A)||0.7µL||Pcon-pT 181 attenuator(2-SA)||2.1 µL||--||--||--||--||0.5 µL||0.5 µL||up to 5 µL | ||
| + | |- | ||
| + | |experiment||RBS-GFP-DT||1.0 µL||Pcon antisense(E-1A)||0.5 µL||spinach-DT(1-2)||1.0 µL||Pcon-GFP-DT(1-2)||0.8 µL||--||--||--||--||0.5 µL||0.5 µL||up to 5 µL | ||
| + | |- | ||
| + | |experiment||pSB1C3(EcoRI&SpeI)||1.8 µL||Pcon-pT181 antisense(E-1A)||0.8µL||spinach-DT(1-2)||1.0µL||Pcon-GFP-DT(2-SA)||0.8 µL||--||--||--||--||0.5 µL||0.5 µL||up to 5 µL | ||
| + | |- | ||
| + | |experiment||pSB1C3(EcoRI&SpeI)||1.8 µL||Pcon-tet aptamer-DT(E-1A)||3.0 µL||Ptet-pT181 antisense(1-3)||1.5 µL||spinach-DT(3-2)||1.5 µL||Pcon-GFP-DT(2-5A)||0.8 µL||--||--||0.5 µL||0.5 µL||up to 5 µL | ||
| + | |} | ||
| + | </div> | ||
| + | |||
| + | ===Liquid Culture=== | ||
| + | <div class="experiment"> | ||
| + | <span class="author">No name</span> | ||
| + | {| class="wikitable" | ||
| + | !Sample||medium | ||
| + | |- | ||
| + | |Pcon-GFP-DT||Amp | ||
| + | |- | ||
| + | |RBS-GFP-DT||CP | ||
| + | |- | ||
| + | |Pcon-pT181 attenuator-DT||Amp | ||
| + | |- | ||
| + | |Pcon-tet aptamer-DT||Amp | ||
| + | |- | ||
| + | |Pcon-spinach-DT||Amp | ||
| + | |- | ||
| + | |Pcon-pT181 antisense-spinach-DT||Amp | ||
| + | |- | ||
| + | |spinach-DT||CP | ||
| + | |} | ||
| + | </div> | ||
| + | |||
| + | ===BSAI Digestion=== | ||
| + | <div class="experiment"> | ||
| + | <span class="author">No name</span> | ||
| + | {| class="wikitable" | ||
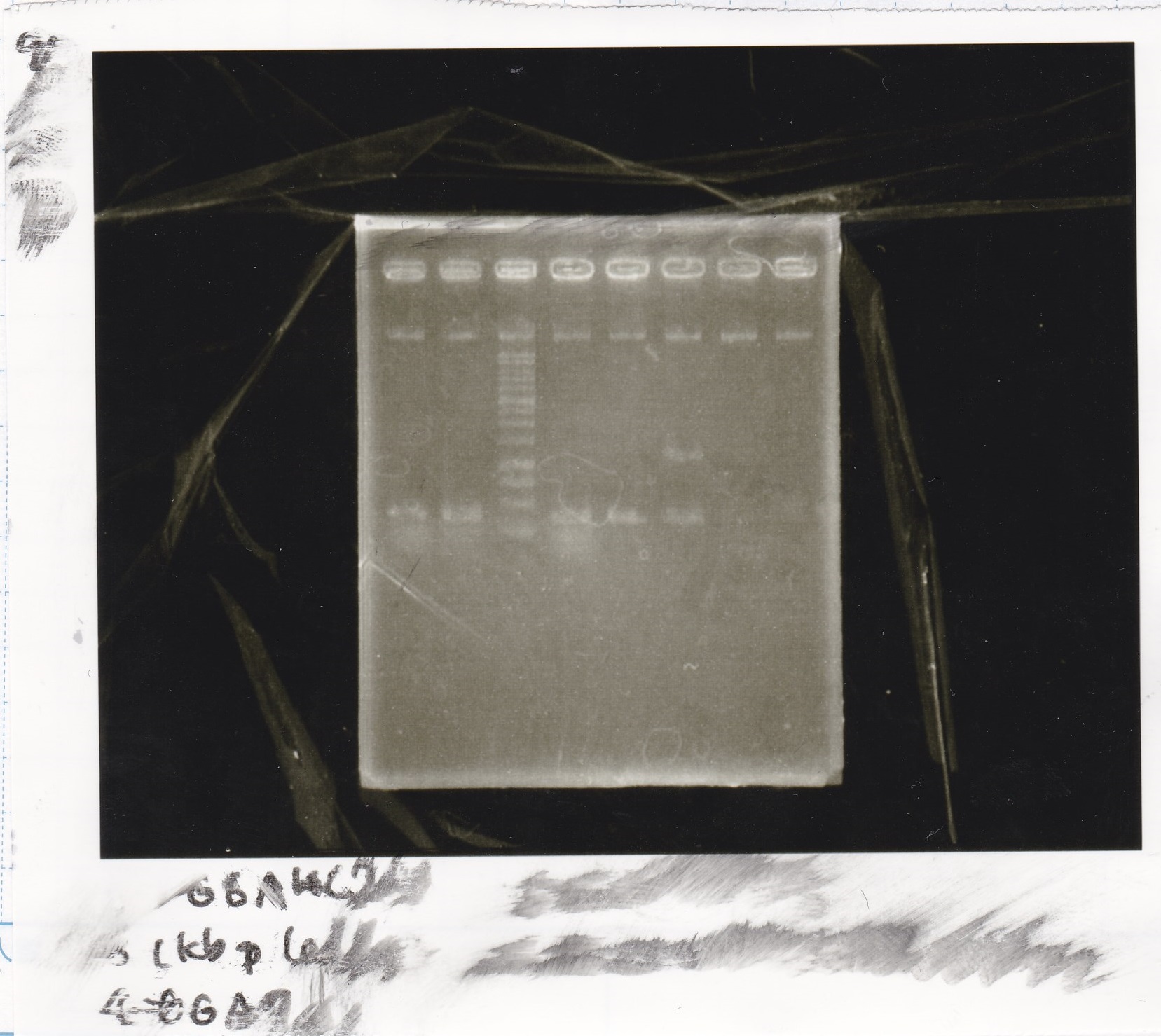
| + | ! ||DNA||MilliQ||BSAI-HF||10*cut smart||total | ||
| + | |- | ||
| + | |Pcon||7 µL||10.7 µL||0.3 µL||2 µL||20 µL | ||
| + | |- | ||
| + | |RBS-luxR-DT||10 µL||7 µL||0.3 µL||2 µL||20 µL | ||
| + | |} | ||
| + | </div> | ||
| + | [[File:Igku_0926_E4.jpg]]<br> | ||
| + | |||
| + | ===Colony PCR=== | ||
| + | <div class="experiment"> | ||
| + | <span class="author">Tatsui</span> | ||
| + | {| class="wikitable" | ||
| + | !Sample||base pair | ||
| + | |- | ||
| + | |GGA-1 (9~16)||3177 | ||
| + | |- | ||
| + | |GGA-2 (9~16)||3189 | ||
| + | |- | ||
| + | |GGA-3 (9~21)||2989 | ||
| + | |- | ||
| + | |GGA-4 (9~16)||2939 | ||
| + | |- | ||
| + | |GGA-6 (1)||3359 | ||
| + | |- | ||
| + | |GGA-16(2~4)||2939 | ||
| + | |} | ||
| + | </div> | ||
| + | {| class="wikitable" | ||
| + | !PreDenature||Denature||Annealing||Extension||cycle | ||
| + | |- | ||
| + | |94°C||94°C||55°C||68°C||-- | ||
| + | |- | ||
| + | |5min||30s||30s||3min30s||30cycles | ||
| + | |} | ||
| + | |||
| + | ===Electrophoresis=== | ||
| + | <div class="experiment"> | ||
| + | <span class="author">No name</span> | ||
{| class="wikitable" | {| class="wikitable" | ||
!Lane||Sample||Enzyme1||Enzyme2 | !Lane||Sample||Enzyme1||Enzyme2 | ||
|- | |- | ||
| - | |1|| | + | |1||GGA-1 (9)||--||-- |
|- | |- | ||
| - | |2|| | + | |2||GGA-1 (10)||--||-- |
|- | |- | ||
| - | |3|| | + | |3||GGA-1 (11)||--||-- |
|- | |- | ||
| - | |4|| | + | |4||GGA-1 (12)||--||-- |
|- | |- | ||
| - | |5|| | + | |5||GGA-1 (13)||--||-- |
|- | |- | ||
| - | |6|| | + | |6||GGA-1 (14)||--||-- |
|- | |- | ||
| - | |7|| | + | |7||GGA-1 (15)||--||-- |
|- | |- | ||
| - | |8|| | + | |8||GGA-1 (16)||--||-- |
| + | |- | ||
| + | |9||1kbp ladder||--||-- | ||
| + | |- | ||
| + | |10||GGA-2 (9)||--||-- | ||
| + | |- | ||
| + | |11||GGA-2 (10)||--||-- | ||
| + | |- | ||
| + | |12||GGA-2 (11)||--||-- | ||
| + | |- | ||
| + | |13||GGA-2 (12)||--||-- | ||
| + | |- | ||
| + | |14||GGA-2 (13)||--||-- | ||
| + | |- | ||
| + | |15||GGA-2 (14)||--||-- | ||
| + | |- | ||
| + | |16||GGA-2 (15)||--||-- | ||
| + | |- | ||
| + | |17||GGA-2 (16)||--||-- | ||
|} | |} | ||
| - | [[File: | + | [[File:Igku_0926_E6.jpg]]<br> |
| + | |||
| + | {| class="wikitable" | ||
| + | !Lane||Sample||Enzyme1||Enzyme2 | ||
| + | |- | ||
| + | |1||GGA-4 (9)||--||-- | ||
| + | |- | ||
| + | |2||GGA-4 (10)||--||-- | ||
| + | |- | ||
| + | |3||GGA-4 (11)||--||-- | ||
| + | |- | ||
| + | |4||GGA-4 (12)||--||-- | ||
| + | |- | ||
| + | |5||GGA-4 (13)||--||-- | ||
| + | |- | ||
| + | |6||GGA-4 (14)||--||-- | ||
| + | |- | ||
| + | |7||GGA-4 (15)||--||-- | ||
| + | |- | ||
| + | |8||GGA-4 (16)||--||-- | ||
| + | |- | ||
| + | |9||1kbp ladder||--||-- | ||
| + | |- | ||
| + | |10||GGA-6 (1)||--||-- | ||
| + | |- | ||
| + | |11||GGA-16 (2)||--||-- | ||
| + | |- | ||
| + | |12||GGA-16 (3)||--||-- | ||
| + | |- | ||
| + | |13||GGA-16 (4)||--||-- | ||
| + | |} | ||
| + | [[File:Igku_0926_E7.jpg]]<br> | ||
| + | |||
| + | {| class="wikitable" | ||
| + | !Lane||Sample||Enzyme1||Enzyme2 | ||
| + | |- | ||
| + | |1||GGA-3 (9)||--||-- | ||
| + | |- | ||
| + | |2||GGA-3 (10)||--||-- | ||
| + | |- | ||
| + | |3||GGA-3 (11)||--||-- | ||
| + | |- | ||
| + | |4||GGA-3 (12)||--||-- | ||
| + | |- | ||
| + | |5||1kbp ladder||--||-- | ||
| + | |- | ||
| + | |6||GGA-3 (13)||--||-- | ||
| + | |- | ||
| + | |7||GGA-3 (15)||--||-- | ||
| + | |- | ||
| + | |8||GGA-3 (14)||--||-- | ||
| + | |} | ||
| + | |||
| + | {| class="wikitable" | ||
| + | !Lane||Sample||Enzyme1||Enzyme2 | ||
| + | |- | ||
| + | |1||GGA-3 (17)||--||-- | ||
| + | |- | ||
| + | |2||GGA-3 (18)||--||-- | ||
| + | |- | ||
| + | |3||GGA-3 (19)||--||-- | ||
| + | |- | ||
| + | |4||1kbp ladder||--||-- | ||
| + | |- | ||
| + | |5||GGA-3 (20)||--||-- | ||
| + | |- | ||
| + | |6||GGA-3 (21)||--||-- | ||
| + | |- | ||
| + | |7||GGA-3 (16)||--||-- | ||
| + | |} | ||
| + | [[File:Igku_0926_E8.jpg]]<br> | ||
</div> | </div> | ||
| + | ===Electrophoresis=== | ||
| + | <div class="experiment"> | ||
| + | <span class="author">No name</span> | ||
{| class="wikitable" | {| class="wikitable" | ||
!Lane||Sample||Enzyme1||Enzyme2 | !Lane||Sample||Enzyme1||Enzyme2 | ||
|- | |- | ||
| - | |1|| | + | |1||RBS-GFP-DT(RNA)||--||-- |
|- | |- | ||
| - | |2|| | + | |2||1kbp ladder||--||-- |
|- | |- | ||
| - | |3|| | + | |3||Pcon-RBS-GFP-DT(RNA)||--||-- |
| + | |} | ||
| + | [[File:Igku_0926_E9.jpg]]<br> | ||
| + | </div> | ||
| + | |||
| + | ===Liquid Culture=== | ||
| + | <div class="experiment"> | ||
| + | <span class="author">Hirano</span> | ||
| + | {| class="wikitable" | ||
| + | !Sample||medium | ||
|- | |- | ||
| - | | | + | |Pcon-GFP-DT||-- |
|- | |- | ||
| - | | | + | |RBS-GFP-DT||-- |
|- | |- | ||
| - | | | + | |Pcon-tet aptamer-DT||-- |
| + | |- | ||
| + | |Pcon-spinach-DT||-- | ||
| + | |- | ||
| + | |Pcon-pT181 antisense-spinach-DT||-- | ||
| + | |- | ||
| + | |spinach-DT||-- | ||
| + | |} | ||
| + | </div> | ||
| + | |||
| + | ===Colony PCR=== | ||
| + | <div class="experiment"> | ||
| + | <span class="author">Hirano</span> | ||
| + | {| class="wikitable" | ||
| + | !Sample||base pair | ||
| + | |- | ||
| + | |9/25 Pcon-pH181 attenuator+RBS-GFP-DT-1||-- | ||
| + | |- | ||
| + | |9/25 Pcon-pH181 attenuator+RBS-GFP-DT-2||-- | ||
| + | |- | ||
| + | |9/25 Pcon-pH181 attenuator+RBS-GFP-DT-3||-- | ||
| + | |- | ||
| + | |9/25 Pcon-pH181 attenuator+RBS-GFP-DT-4||-- | ||
| + | |- | ||
| + | |9/24 Ptet-RBS-GFP-DT-1||-- | ||
| + | |} | ||
| + | {| class="wikitable" | ||
| + | !PreDenature||Denature||Annealing||Extension||cycle | ||
| + | |- | ||
| + | |94°C||94°C||55°C||68°C||-- | ||
| + | |- | ||
| + | |5min||30s||30s||1min40s||30cycles | ||
|} | |} | ||
| - | |||
</div> | </div> | ||
===Ligasion(Golden Gate Assenbly)=== | ===Ligasion(Golden Gate Assenbly)=== | ||
| + | <div class="experiment"> | ||
| + | <span class="author">Hirano</span> | ||
| + | {| class="wikitable" | ||
| + | !state||Vector||Inserter1||Inserter2||Inserter3||Inserter4||Inserter5 | ||
| + | |- | ||
| + | |experiment||GGA2||Pcon-attenuator(E-1A)||Pcon-tetR-DT(1-2A)||Ptet(2-S)||RBS-GFP-DT(EcoRI&XbaI)|| | ||
| + | |- | ||
| + | |experiment||GGA3||Pcon-tetap-DT(E-1A)||Pcon-tetR-DT(1-2A)||Ptet(2-S)||RBS-GFP-DT(EcoRI&XbaI)|| | ||
| + | |- | ||
| + | |experiment||GGA4||Pcon-spi-DT(E-1A)||Pcon-tetR-DT(1-2A)||Ptet(2-S)||RBS-GFP-DT(EcoRI&XbaI)|| | ||
| + | |- | ||
| + | |experiment||GGA5||Pcon-antisense(E-1A)||DT(1-3)||Pcon-tetR-DT(3-2A)||Ptet(2-S)||RBS-GFP-DT(EcoRI&XbaI) | ||
| + | |- | ||
| + | |experiment||GGA6||Pcon-attenuator(E-1A)||tetap-DT(1-3)||Pcon-tetR-DT(3-2A)||Ptet(2-S)||RBS-GFP-DT(EcoRI&XbaI) | ||
| + | |- | ||
| + | |experiment||GGA7||Pcon-tetR-DT(E-2A)||Ptet(2-S)||RBS-GFP-DT(EcoRI&XbaI)|||| | ||
| + | |} | ||
| + | {| class="wikitable" | ||
| + | !PreDenature||Denature||Annealing||Extension||cycle | ||
| + | |- | ||
| + | |37°C||16°C||50°C||80°C||-- | ||
| + | |- | ||
| + | |3min||4min||5min||5min||25cycles | ||
| + | |} | ||
| + | </div> | ||
| + | |||
| + | ===RNA Extraction=== | ||
| + | <div class="experiment"> | ||
| + | <span class="author">Nakamoto</span> | ||
| + | {| class="wikitable" | ||
| + | !Sample | ||
| + | |- | ||
| + | |Pcon-Spinach-DT | ||
| + | |- | ||
| + | |Pcon-tetRaptamer-DT | ||
| + | |- | ||
| + | |Pcon-attenuator-DT | ||
| + | |- | ||
| + | |Spinach-DT | ||
| + | |- | ||
| + | |Pcon-antisense-Spinach-DT | ||
| + | |} | ||
| + | </div> | ||
| + | |||
| + | ===Liquid Culture=== | ||
| + | <div class="experiment"> | ||
| + | <span class="author">Kozima</span> | ||
| + | {| class="wikitable" | ||
| + | !Sample | ||
| + | |- | ||
| + | |Pcon-attemuater-DT | ||
| + | |- | ||
| + | |Pcon-aptamer-DT | ||
| + | |- | ||
| + | |Pcon-spinach-DT | ||
| + | |- | ||
| + | |Pcon-RBS-tetR-DT | ||
| + | |- | ||
| + | |Pcon-antisense-spinach-DT(Master 1) | ||
| + | |- | ||
| + | |Pcon-antisense | ||
| + | |} | ||
| + | </div> | ||
| + | |||
| + | ===Colony PCR=== | ||
| + | <div class="experiment"> | ||
| + | <span class="author">Nakamoto Kozima</span> | ||
| + | {| class="wikitable" | ||
| + | !Sample||base pair | ||
| + | |- | ||
| + | |9/25 9/19pSB1C3&9/3PT181 attenuator(1~3)||601bp | ||
| + | |- | ||
| + | |9/25 aptamer12_1R-DT&9/24Pcon-pT181 attenuator(1~4)||859bp | ||
| + | |- | ||
| + | |Pcon-attenuator-aptamer-DT(1)||859bp | ||
| + | |} | ||
| + | {| class="wikitable" | ||
| + | !PreDenature||Denature||Annealing||Extension||cycle | ||
| + | |- | ||
| + | | 94°C|| 94°C|| 55°C|| 65°C||-- | ||
| + | |- | ||
| + | |5min||30sec ||30sec ||54sec ||30 | ||
| + | |} | ||
| + | </div> | ||
| + | |||
| + | ===Electrophoresis=== | ||
<div class="experiment"> | <div class="experiment"> | ||
<span class="author">No name</span> | <span class="author">No name</span> | ||
{| class="wikitable" | {| class="wikitable" | ||
| - | ! | + | !Lane||Sample||Enzyme1||Enzyme2 |
|- | |- | ||
| - | | | + | |1||1kbp ladder||--||-- |
|- | |- | ||
| - | | | + | |2||9/25 9/23 1C3&9/3attenuator||--||-- |
|- | |- | ||
| - | | | + | |3||9/25 9/23 1C3&9/3attenuator||--||-- |
|- | |- | ||
| - | | | + | |4||9/25 9/23 1C3&9/3attenuator||--||-- |
|- | |- | ||
| - | | | + | |5||9/25aptamer-DT&9/24Pcon-attenuator||--||-- |
|- | |- | ||
| + | |6||9/25aptamer-DT&9/24Pcon-attenuator||--||-- | ||
| + | |- | ||
| + | |7||9/25aptamer-DT&9/24Pcon-attenuator||--||-- | ||
| + | |- | ||
| + | |8||9/24 Pcon attenuator-aptamer-DT(1)||--||-- | ||
| + | |- | ||
| + | |9||9/25aptamer-DT&9/24Pcon-attenuator||--||-- | ||
|} | |} | ||
| + | [[File:Igku_0926_E10.jpg]]<br> | ||
</div> | </div> | ||
| + | |||
| + | ===Plating=== | ||
| + | <div class="experiment"> | ||
| + | <span class="author">Hirano</span> | ||
| + | </div> | ||
| + | {| class="wikitable" | ||
| + | !Sample||Use plate | ||
| + | |- | ||
| + | |9/26 GGA2 ||LB(CP) | ||
| + | |- | ||
| + | |9/26 GGA3 ||LB(CP) | ||
| + | |- | ||
| + | |9/26 GGA4||LB(CP) | ||
| + | |- | ||
| + | |9/26 GGA5 ||LB(CP) | ||
| + | |- | ||
| + | |9/26 GGA6 ||LB(CP) | ||
| + | |- | ||
| + | |9/26 GGA7 ||LB(CP) | ||
| + | |- | ||
| + | |9/26 GGA11 ||LB(CP) | ||
| + | |- | ||
| + | |9/26 GGA312||LB(CP) | ||
| + | |- | ||
| + | |9/26 GGA13 ||LB(CP) | ||
| + | |- | ||
| + | |9/26 GGA14||LB(CP) | ||
| + | |- | ||
| + | |9/26 GGA15 ||LB(CP) | ||
| + | |- | ||
| + | |9/26 GGA20||LB(CP) | ||
| + | |- | ||
| + | |9/26 GGA23 ||LB(CP) | ||
| + | |- | ||
| + | |9/26 GGA24||LB(CP) | ||
| + | |- | ||
| + | |9/26 Ptet+RBS-GFP-DT ||LB(CP) | ||
| + | |} | ||
| + | incubate 37°C | ||
| + | |||
| + | ===Restriction Enzyme Digestion=== | ||
| + | <div class="experiment"> | ||
| + | <span class="author">Kojima</span> | ||
| + | {| class="wikitable" | ||
| + | !||9/24 pSB1C3||EcoRI||SpeI||buffer||MilliQ||total | ||
| + | |- | ||
| + | |2cuts||11µL||1µL||1µL||3µL||14µL||30µL | ||
| + | |- | ||
| + | |NC||0.5µL||0µL||0µL||1µL||8.5µL||10µL | ||
| + | |} | ||
| + | |||
| + | {| class="wikitable" | ||
| + | !||9/24 Pcon-pT181attenuator-aptamer12-1R-DT||EcoRI||SpeI||buffer||MilliQ||total | ||
| + | |- | ||
| + | |2cuts||8.7µL||1µL||1µL||3µL||16.3µL||30µL | ||
| + | |- | ||
| + | |NC||0.4µL||0µL||0µL||1µL||8.6µL||10µL | ||
| + | |} | ||
| + | {| class="wikitable" | ||
| + | !||9/24 pT181attenuator||EcoRI||SpeI||buffer||MilliQ||total | ||
| + | |- | ||
| + | |2cuts||3.1µL||1µL||1µL||3µL||21.9µL||30µL | ||
| + | |- | ||
| + | |NC||0.2µL||0µL||0µL||1µL||8.8µL||10µL | ||
| + | |} | ||
| + | {| class="wikitable" | ||
| + | !||8/17 RBS-GFP-DT||EcoRI||BSA ||buffer||MilliQ||total | ||
| + | |- | ||
| + | |1cut||16.4µL||1µL||3µL||3µL||6.6µL||30µL | ||
| + | |- | ||
| + | |NC||0.4µL||0µL||1µL||1µL||7.6µL||10µL | ||
| + | |} | ||
| + | {| class="wikitable" | ||
| + | !||9/17 pSB4K5||EcoRI||PstI||BSA ||buffer||MilliQ||total | ||
| + | |- | ||
| + | |2cuts||8.5µL||1µL||1µL||3µL||3µL||13.5µL||30µL | ||
| + | |- | ||
| + | |NC||0.4µL||0µL||0µL||1µL||1µL||7.6µL||10µL | ||
| + | |} | ||
| + | |||
| + | {| class="wikitable" | ||
| + | !||9/14 pSB4K5||EcoRI||PstI||BSA ||buffer||MilliQ||total | ||
| + | |- | ||
| + | |2cuts||7.3µL||1µL||1µL||3µL||3µL||14.7µL||30µL | ||
| + | |- | ||
| + | |NC||0.4µL||0µL||0µL||1µL||1µL||7.6µL||10µL | ||
| + | |} | ||
| + | {| class="wikitable" | ||
| + | !||9/22 Pcon-pT181attenuator-DT||EcoRI||SpeI ||buffer||MilliQ||total | ||
| + | |- | ||
| + | |2cuts||5.4µL||1µL||1µL||3µL||19.6µL||30µL | ||
| + | |- | ||
| + | |NC||0.3µL||0µL||0µL||1µL||8.7µL||10µL | ||
| + | |} | ||
| + | {| class="wikitable" | ||
| + | !||8/7 RBS-GFP-DT 1||XbaI||PstI||BSA ||buffer||MilliQ||total | ||
| + | |- | ||
| + | |2cuts||8.1µL||1µL||1µL||3µL||3µL||13.9µL||30µL | ||
| + | |- | ||
| + | |NC||0.4µL||0µL||0µL||1µL||1µL||7.6µL||10µL | ||
| + | |} | ||
| + | {| class="wikitable" | ||
| + | !||9/26 RBS-GFP-DT 1||XbaI ||BSA ||buffer||MilliQ||total | ||
| + | |- | ||
| + | |1cut||8.1µL||1µL||3µL||3µL||14.9µL||30µL | ||
| + | |} | ||
| + | {| class="wikitable" | ||
| + | !||9/16 Pλ-luxI||EcoRI||PstI||BSA ||buffer||MilliQ||total | ||
| + | |- | ||
| + | |2cuts||14.7µL||1µL||1µL||3µL||3µL||7.3µL||30µL | ||
| + | |- | ||
| + | |NC||0.7µL||0µL||0µL||1µL||1µL||7.3µL||10µL | ||
| + | |} | ||
| + | {| class="wikitable" | ||
| + | !||9/21 Ptet||EcoRI||SpeI ||buffer||MilliQ||total | ||
| + | |- | ||
| + | |2cuts||14.2µL||1µL||1µL||3µL||10.8µL||30µL | ||
| + | |- | ||
| + | |NC||0.7µL||0µL||0µL||1µL||8.3µL||10µL | ||
| + | |} | ||
| + | {| class="wikitable" | ||
| + | !||9/24 Pcon-pT181attenuator-aptamer12-1R-DT||XbaI||PstI||BSA ||buffer||MilliQ||total | ||
| + | |- | ||
| + | |2cuts||8.7µL||1µL||1µL||3µL||3µL||13.3µL||30µL | ||
| + | |- | ||
| + | |NC||0.4µL||0µL||0µL||1µL||1µL||7.6µL||10µL | ||
| + | |} | ||
| + | |||
| + | </div> | ||
| + | |||
| + | ===Electrophoresis=== | ||
| + | <div class="experiment"> | ||
| + | <span class="author">Kojima</span> | ||
| + | {| class="wikitable" | ||
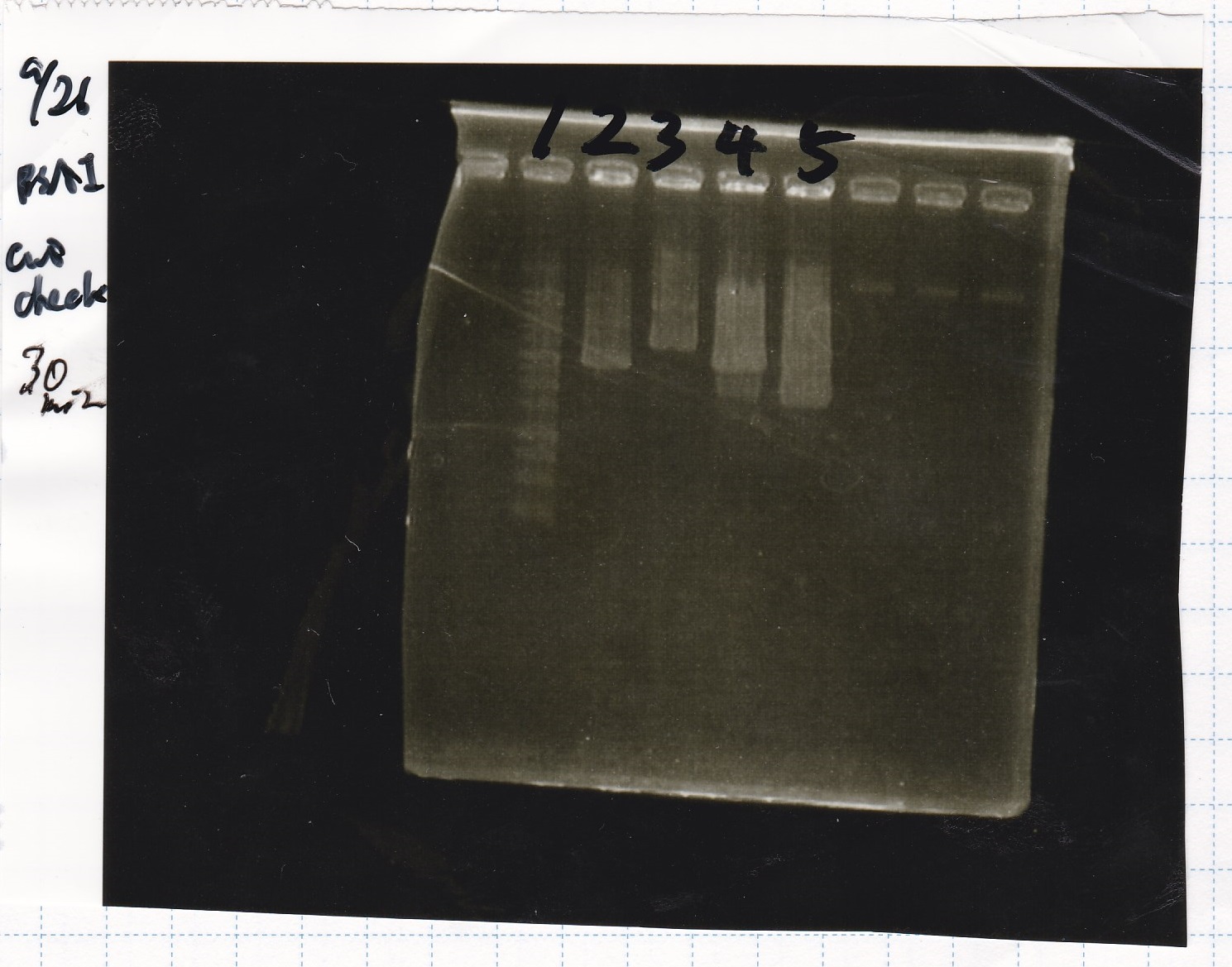
| + | !Lane||Sample|| Enzyme1|| Enzyme2 | ||
| + | |- | ||
| + | |1||pSB1C3||EcoRI||SpeI | ||
| + | |- | ||
| + | |2||pSB1C3||--||-- | ||
| + | |- | ||
| + | |3||Pcon-pT181attenuator-aptamer12-1R-DT||EcoRI||SpeI | ||
| + | |- | ||
| + | |4||Pcon-pT181attenuator-aptamer12-1R-DT||--||-- | ||
| + | |- | ||
| + | |5||1kb ladder||--||-- | ||
| + | |- | ||
| + | |6||pT181attenuator||EcoRI||SpeI | ||
| + | |- | ||
| + | |7||pT181attenuator||--||-- | ||
| + | |- | ||
| + | |8||RBS-GFP-DT||EcoRI||-- | ||
| + | |- | ||
| + | |9||RBS-GFP-DT||--||-- | ||
| + | |} | ||
| + | [[File:Igku Sep26 Electrophoresis(E11) 1.jpg]]<br> | ||
Latest revision as of 18:26, 27 September 2013
Sep 26
Electrophoresis
| Lane | Sample | Enzyme1 | Enzyme2 |
|---|---|---|---|
| 1 | GGA1-1 | -- | -- |
| 2 | GGA1-2 | -- | -- |
| 3 | GGA1-3 | -- | -- |
| 4 | GGA1-4 | -- | -- |
| 5 | GGA1-5 | -- | -- |
| 6 | GGA1-6 | -- | -- |
| 7 | GGA1-7 | -- | -- |
| 8 | GGA1-8 | -- | -- |
| 9 | 1kbp ladder | -- | -- |
| 10 | GGA2-1 | -- | -- |
| 11 | GGA2-2 | -- | -- |
| 12 | GGA2-3 | -- | -- |
| 13 | GGA2-4 | -- | -- |
| 14 | GGA2-5 | -- | -- |
| 15 | GGA2-6 | -- | -- |
| 16 | GGA2-7 | -- | -- |
| 17 | GGA2-8 | -- | -- |
| Lane | Sample | Enzyme1 | Enzyme2 |
|---|---|---|---|
| 1 | GGA3-1 | -- | -- |
| 2 | GGA3-2 | -- | -- |
| 3 | GGA3-3 | -- | -- |
| 4 | 1kbp ladder | -- | -- |
| 5 | GGA3-4 | -- | -- |
| 6 | GGA3-5 | -- | -- |
| 7 | GGA3-6 | -- | -- |
| 8 | GGA3-7 | -- | -- |
| Lane | Sample | Enzyme1 | Enzyme2 |
|---|---|---|---|
| 1 | GGA3-8 | -- | -- |
| 2 | GGA4-1 | -- | -- |
| 3 | GGA4-2 | -- | -- |
| 4 | GGA4-3 | -- | -- |
| 5 | 1kbp ladder | -- | -- |
| 6 | GGA4-4 | -- | -- |
| 7 | GGA4-5 | -- | -- |
| 8 | GGA4-6 | -- | -- |
| Lane | Sample | Enzyme1 | Enzyme2 |
|---|---|---|---|
| 1 | GGA4-7 | -- | -- |
| 2 | GGA4-8 | -- | -- |
| 3 | 1kbp ladder | -- | -- |
| 4 | GGA7-1 | -- | -- |
| 5 | GGA16-1 | -- | -- |
| 6 | NC(RBS-GFP-DT) | -- | -- |
Ligasion(Golden Gate Assenbly)
| state | Vector | Inserter1 | Inserter2 | Inserter3 | Inserter4 | Inserter5 | (NEB)T4 ligase | (NEB)10*T4 ligase | MilliQ | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| experiment | RBS-GFP-DT | 1.0 µL | Pcon-pT181 antisense(E-2A) | 0.6µL | DT(2-1) | 0.5µL | Pcon-pT181 attenuator(1-SA) | 2.1 µL | -- | -- | -- | -- | 0.5 µL | 0.5 µL | up to 5 µL |
| experiment | pSB1C3(EcoRI&SpeI) | 1.8 µL | Pcon-pT181 antisense(E-2A) | 0.9 µL | DT(2-1) | 0.5 µL | Pcon-GFP-DT(1-SA) | 1.5 µL | -- | -- | -- | -- | 0.5 µL | 0.5 µL | up to 5 µL |
| experiment | RBS-GFP-DT | 1.0 µL | Ptet(E-0) | 0.7µL | Pcon pT181 attenuator(0-1A) | 0.3µL | tet aptamer(1-3) | 0.3 µL | Pcon-tetR-DT(3-2A) | 1.1 µL | Ptet(2-5) | 0.3 µL | 0.5 µL | 0.5 µL | up to 5 µL |
| experiment | RBS-GFP-DT | 1.0 µL | Ptet(E-0) | 0.7 µL | Pcon-pT181 attenuator-DT(0-1A) | 1.0 µL | Pcon-tetR-DT(1-2A) | 1.0 µL | Ptet(2-5) | 0.3µL | -- | -- | 0.5 µL | 0.5 µL | up to 5 µL |
| experiment | RBS-GFP-DT | 1.0 µL | Pcon-pT181 attenuator-tetR-DT(E-1A) | 1.0 µL | Pcon antisense-spinach-DT(1-2A) | 0.7µL | Pcon-pT 181 attenuator(2-SA) | 2.1 µL | -- | -- | -- | -- | 0.5 µL | 0.5 µL | up to 5 µL |
| experiment | RBS-GFP-DT | 1.0 µL | Pcon antisense(E-1A) | 0.5 µL | spinach-DT(1-2) | 1.0 µL | Pcon-GFP-DT(1-2) | 0.8 µL | -- | -- | -- | -- | 0.5 µL | 0.5 µL | up to 5 µL |
| experiment | pSB1C3(EcoRI&SpeI) | 1.8 µL | Pcon-pT181 antisense(E-1A) | 0.8µL | spinach-DT(1-2) | 1.0µL | Pcon-GFP-DT(2-SA) | 0.8 µL | -- | -- | -- | -- | 0.5 µL | 0.5 µL | up to 5 µL |
| experiment | pSB1C3(EcoRI&SpeI) | 1.8 µL | Pcon-tet aptamer-DT(E-1A) | 3.0 µL | Ptet-pT181 antisense(1-3) | 1.5 µL | spinach-DT(3-2) | 1.5 µL | Pcon-GFP-DT(2-5A) | 0.8 µL | -- | -- | 0.5 µL | 0.5 µL | up to 5 µL |
Liquid Culture
| Sample | medium |
|---|---|
| Pcon-GFP-DT | Amp |
| RBS-GFP-DT | CP |
| Pcon-pT181 attenuator-DT | Amp |
| Pcon-tet aptamer-DT | Amp |
| Pcon-spinach-DT | Amp |
| Pcon-pT181 antisense-spinach-DT | Amp |
| spinach-DT | CP |
BSAI Digestion
| DNA | MilliQ | BSAI-HF | 10*cut smart | total | |
|---|---|---|---|---|---|
| Pcon | 7 µL | 10.7 µL | 0.3 µL | 2 µL | 20 µL |
| RBS-luxR-DT | 10 µL | 7 µL | 0.3 µL | 2 µL | 20 µL |
Colony PCR
| Sample | base pair |
|---|---|
| GGA-1 (9~16) | 3177 |
| GGA-2 (9~16) | 3189 |
| GGA-3 (9~21) | 2989 |
| GGA-4 (9~16) | 2939 |
| GGA-6 (1) | 3359 |
| GGA-16(2~4) | 2939 |
| PreDenature | Denature | Annealing | Extension | cycle |
|---|---|---|---|---|
| 94°C | 94°C | 55°C | 68°C | -- |
| 5min | 30s | 30s | 3min30s | 30cycles |
Electrophoresis
| Lane | Sample | Enzyme1 | Enzyme2 |
|---|---|---|---|
| 1 | GGA-1 (9) | -- | -- |
| 2 | GGA-1 (10) | -- | -- |
| 3 | GGA-1 (11) | -- | -- |
| 4 | GGA-1 (12) | -- | -- |
| 5 | GGA-1 (13) | -- | -- |
| 6 | GGA-1 (14) | -- | -- |
| 7 | GGA-1 (15) | -- | -- |
| 8 | GGA-1 (16) | -- | -- |
| 9 | 1kbp ladder | -- | -- |
| 10 | GGA-2 (9) | -- | -- |
| 11 | GGA-2 (10) | -- | -- |
| 12 | GGA-2 (11) | -- | -- |
| 13 | GGA-2 (12) | -- | -- |
| 14 | GGA-2 (13) | -- | -- |
| 15 | GGA-2 (14) | -- | -- |
| 16 | GGA-2 (15) | -- | -- |
| 17 | GGA-2 (16) | -- | -- |
| Lane | Sample | Enzyme1 | Enzyme2 |
|---|---|---|---|
| 1 | GGA-4 (9) | -- | -- |
| 2 | GGA-4 (10) | -- | -- |
| 3 | GGA-4 (11) | -- | -- |
| 4 | GGA-4 (12) | -- | -- |
| 5 | GGA-4 (13) | -- | -- |
| 6 | GGA-4 (14) | -- | -- |
| 7 | GGA-4 (15) | -- | -- |
| 8 | GGA-4 (16) | -- | -- |
| 9 | 1kbp ladder | -- | -- |
| 10 | GGA-6 (1) | -- | -- |
| 11 | GGA-16 (2) | -- | -- |
| 12 | GGA-16 (3) | -- | -- |
| 13 | GGA-16 (4) | -- | -- |
| Lane | Sample | Enzyme1 | Enzyme2 |
|---|---|---|---|
| 1 | GGA-3 (9) | -- | -- |
| 2 | GGA-3 (10) | -- | -- |
| 3 | GGA-3 (11) | -- | -- |
| 4 | GGA-3 (12) | -- | -- |
| 5 | 1kbp ladder | -- | -- |
| 6 | GGA-3 (13) | -- | -- |
| 7 | GGA-3 (15) | -- | -- |
| 8 | GGA-3 (14) | -- | -- |
| Lane | Sample | Enzyme1 | Enzyme2 |
|---|---|---|---|
| 1 | GGA-3 (17) | -- | -- |
| 2 | GGA-3 (18) | -- | -- |
| 3 | GGA-3 (19) | -- | -- |
| 4 | 1kbp ladder | -- | -- |
| 5 | GGA-3 (20) | -- | -- |
| 6 | GGA-3 (21) | -- | -- |
| 7 | GGA-3 (16) | -- | -- |
Electrophoresis
Liquid Culture
| Sample | medium |
|---|---|
| Pcon-GFP-DT | -- |
| RBS-GFP-DT | -- |
| Pcon-tet aptamer-DT | -- |
| Pcon-spinach-DT | -- |
| Pcon-pT181 antisense-spinach-DT | -- |
| spinach-DT | -- |
Colony PCR
| Sample | base pair |
|---|---|
| 9/25 Pcon-pH181 attenuator+RBS-GFP-DT-1 | -- |
| 9/25 Pcon-pH181 attenuator+RBS-GFP-DT-2 | -- |
| 9/25 Pcon-pH181 attenuator+RBS-GFP-DT-3 | -- |
| 9/25 Pcon-pH181 attenuator+RBS-GFP-DT-4 | -- |
| 9/24 Ptet-RBS-GFP-DT-1 | -- |
| PreDenature | Denature | Annealing | Extension | cycle |
|---|---|---|---|---|
| 94°C | 94°C | 55°C | 68°C | -- |
| 5min | 30s | 30s | 1min40s | 30cycles |
Ligasion(Golden Gate Assenbly)
| state | Vector | Inserter1 | Inserter2 | Inserter3 | Inserter4 | Inserter5 |
|---|---|---|---|---|---|---|
| experiment | GGA2 | Pcon-attenuator(E-1A) | Pcon-tetR-DT(1-2A) | Ptet(2-S) | RBS-GFP-DT(EcoRI&XbaI) | |
| experiment | GGA3 | Pcon-tetap-DT(E-1A) | Pcon-tetR-DT(1-2A) | Ptet(2-S) | RBS-GFP-DT(EcoRI&XbaI) | |
| experiment | GGA4 | Pcon-spi-DT(E-1A) | Pcon-tetR-DT(1-2A) | Ptet(2-S) | RBS-GFP-DT(EcoRI&XbaI) | |
| experiment | GGA5 | Pcon-antisense(E-1A) | DT(1-3) | Pcon-tetR-DT(3-2A) | Ptet(2-S) | RBS-GFP-DT(EcoRI&XbaI) |
| experiment | GGA6 | Pcon-attenuator(E-1A) | tetap-DT(1-3) | Pcon-tetR-DT(3-2A) | Ptet(2-S) | RBS-GFP-DT(EcoRI&XbaI) |
| experiment | GGA7 | Pcon-tetR-DT(E-2A) | Ptet(2-S) | RBS-GFP-DT(EcoRI&XbaI) |
| PreDenature | Denature | Annealing | Extension | cycle |
|---|---|---|---|---|
| 37°C | 16°C | 50°C | 80°C | -- |
| 3min | 4min | 5min | 5min | 25cycles |
RNA Extraction
| Sample |
|---|
| Pcon-Spinach-DT |
| Pcon-tetRaptamer-DT |
| Pcon-attenuator-DT |
| Spinach-DT |
| Pcon-antisense-Spinach-DT |
Liquid Culture
| Sample |
|---|
| Pcon-attemuater-DT |
| Pcon-aptamer-DT |
| Pcon-spinach-DT |
| Pcon-RBS-tetR-DT |
| Pcon-antisense-spinach-DT(Master 1) |
| Pcon-antisense |
Colony PCR
| Sample | base pair |
|---|---|
| 9/25 9/19pSB1C3&9/3PT181 attenuator(1~3) | 601bp |
| 9/25 aptamer12_1R-DT&9/24Pcon-pT181 attenuator(1~4) | 859bp |
| Pcon-attenuator-aptamer-DT(1) | 859bp |
| PreDenature | Denature | Annealing | Extension | cycle |
|---|---|---|---|---|
| 94°C | 94°C | 55°C | 65°C | -- |
| 5min | 30sec | 30sec | 54sec | 30 |
Electrophoresis
| Lane | Sample | Enzyme1 | Enzyme2 |
|---|---|---|---|
| 1 | 1kbp ladder | -- | -- |
| 2 | 9/25 9/23 1C3&9/3attenuator | -- | -- |
| 3 | 9/25 9/23 1C3&9/3attenuator | -- | -- |
| 4 | 9/25 9/23 1C3&9/3attenuator | -- | -- |
| 5 | 9/25aptamer-DT&9/24Pcon-attenuator | -- | -- |
| 6 | 9/25aptamer-DT&9/24Pcon-attenuator | -- | -- |
| 7 | 9/25aptamer-DT&9/24Pcon-attenuator | -- | -- |
| 8 | 9/24 Pcon attenuator-aptamer-DT(1) | -- | -- |
| 9 | 9/25aptamer-DT&9/24Pcon-attenuator | -- | -- |
Plating
| Sample | Use plate |
|---|---|
| 9/26 GGA2 | LB(CP) |
| 9/26 GGA3 | LB(CP) |
| 9/26 GGA4 | LB(CP) |
| 9/26 GGA5 | LB(CP) |
| 9/26 GGA6 | LB(CP) |
| 9/26 GGA7 | LB(CP) |
| 9/26 GGA11 | LB(CP) |
| 9/26 GGA312 | LB(CP) |
| 9/26 GGA13 | LB(CP) |
| 9/26 GGA14 | LB(CP) |
| 9/26 GGA15 | LB(CP) |
| 9/26 GGA20 | LB(CP) |
| 9/26 GGA23 | LB(CP) |
| 9/26 GGA24 | LB(CP) |
| 9/26 Ptet+RBS-GFP-DT | LB(CP) |
incubate 37°C
Restriction Enzyme Digestion
| 9/24 pSB1C3 | EcoRI | SpeI | buffer | MilliQ | total | |
|---|---|---|---|---|---|---|
| 2cuts | 11µL | 1µL | 1µL | 3µL | 14µL | 30µL |
| NC | 0.5µL | 0µL | 0µL | 1µL | 8.5µL | 10µL |
| 9/24 Pcon-pT181attenuator-aptamer12-1R-DT | EcoRI | SpeI | buffer | MilliQ | total | |
|---|---|---|---|---|---|---|
| 2cuts | 8.7µL | 1µL | 1µL | 3µL | 16.3µL | 30µL |
| NC | 0.4µL | 0µL | 0µL | 1µL | 8.6µL | 10µL |
| 9/24 pT181attenuator | EcoRI | SpeI | buffer | MilliQ | total | |
|---|---|---|---|---|---|---|
| 2cuts | 3.1µL | 1µL | 1µL | 3µL | 21.9µL | 30µL |
| NC | 0.2µL | 0µL | 0µL | 1µL | 8.8µL | 10µL |
| 8/17 RBS-GFP-DT | EcoRI | BSA | buffer | MilliQ | total | |
|---|---|---|---|---|---|---|
| 1cut | 16.4µL | 1µL | 3µL | 3µL | 6.6µL | 30µL |
| NC | 0.4µL | 0µL | 1µL | 1µL | 7.6µL | 10µL |
| 9/17 pSB4K5 | EcoRI | PstI | BSA | buffer | MilliQ | total | |
|---|---|---|---|---|---|---|---|
| 2cuts | 8.5µL | 1µL | 1µL | 3µL | 3µL | 13.5µL | 30µL |
| NC | 0.4µL | 0µL | 0µL | 1µL | 1µL | 7.6µL | 10µL |
| 9/14 pSB4K5 | EcoRI | PstI | BSA | buffer | MilliQ | total | |
|---|---|---|---|---|---|---|---|
| 2cuts | 7.3µL | 1µL | 1µL | 3µL | 3µL | 14.7µL | 30µL |
| NC | 0.4µL | 0µL | 0µL | 1µL | 1µL | 7.6µL | 10µL |
| 9/22 Pcon-pT181attenuator-DT | EcoRI | SpeI | buffer | MilliQ | total | |
|---|---|---|---|---|---|---|
| 2cuts | 5.4µL | 1µL | 1µL | 3µL | 19.6µL | 30µL |
| NC | 0.3µL | 0µL | 0µL | 1µL | 8.7µL | 10µL |
| 8/7 RBS-GFP-DT 1 | XbaI | PstI | BSA | buffer | MilliQ | total | |
|---|---|---|---|---|---|---|---|
| 2cuts | 8.1µL | 1µL | 1µL | 3µL | 3µL | 13.9µL | 30µL |
| NC | 0.4µL | 0µL | 0µL | 1µL | 1µL | 7.6µL | 10µL |
| 9/26 RBS-GFP-DT 1 | XbaI | BSA | buffer | MilliQ | total | |
|---|---|---|---|---|---|---|
| 1cut | 8.1µL | 1µL | 3µL | 3µL | 14.9µL | 30µL |
| 9/16 Pλ-luxI | EcoRI | PstI | BSA | buffer | MilliQ | total | |
|---|---|---|---|---|---|---|---|
| 2cuts | 14.7µL | 1µL | 1µL | 3µL | 3µL | 7.3µL | 30µL |
| NC | 0.7µL | 0µL | 0µL | 1µL | 1µL | 7.3µL | 10µL |
| 9/21 Ptet | EcoRI | SpeI | buffer | MilliQ | total | |
|---|---|---|---|---|---|---|
| 2cuts | 14.2µL | 1µL | 1µL | 3µL | 10.8µL | 30µL |
| NC | 0.7µL | 0µL | 0µL | 1µL | 8.3µL | 10µL |
| 9/24 Pcon-pT181attenuator-aptamer12-1R-DT | XbaI | PstI | BSA | buffer | MilliQ | total | |
|---|---|---|---|---|---|---|---|
| 2cuts | 8.7µL | 1µL | 1µL | 3µL | 3µL | 13.3µL | 30µL |
| NC | 0.4µL | 0µL | 0µL | 1µL | 1µL | 7.6µL | 10µL |
Electrophoresis
| Lane | Sample | Enzyme1 | Enzyme2 |
|---|---|---|---|
| 1 | pSB1C3 | EcoRI | SpeI |
| 2 | pSB1C3 | -- | -- |
| 3 | Pcon-pT181attenuator-aptamer12-1R-DT | EcoRI | SpeI |
| 4 | Pcon-pT181attenuator-aptamer12-1R-DT | -- | -- |
| 5 | 1kb ladder | -- | -- |
| 6 | pT181attenuator | EcoRI | SpeI |
| 7 | pT181attenuator | -- | -- |
| 8 | RBS-GFP-DT | EcoRI | -- |
| 9 | RBS-GFP-DT | -- | -- |
 "
"