From 2013.igem.org
(Difference between revisions)
|
|
| Line 526: |
Line 526: |
| | !state||colspan="2"|Vector||colspan="2"|Inserter | | !state||colspan="2"|Vector||colspan="2"|Inserter |
| | |- | | |- |
| - | |experiment||Pcon-spin-DT(EcoRI&SpeI)||10.5µg/mL||pSB6A1(EcoRI&SpeI)||8.9µg/mL | + | |experiment||Pcon-spinach-DT(EcoRI&SpeI)||10.5µg/mL||pSB6A1(EcoRI&SpeI)||8.9µg/mL |
| | |- | | |- |
| - | |experiment||Pcon-spin-DT(EcoRI&SpeI)||10.5µg/mL||pSB4K5(EcoRI&SpeI)||21.8µg/mL | + | |experiment||Pcon-spinach-DT(EcoRI&SpeI)||10.5µg/mL||pSB4K5(EcoRI&SpeI)||21.8µg/mL |
| | |- | | |- |
| - | |experiment||Pcon-pT181 anti(SpeI+PstI)||31.3µg/mL||spin-DT(XbaI+PstI)||10.1µg/mL | + | |experiment||Pcon-pT181 anti(SpeI+PstI)||31.3µg/mL||spinach-DT(XbaI+PstI)||10.1µg/mL |
| | |- | | |- |
| | |experiment||Plac(SpeI+PstI)||11.6µg/mL||RBS-lysis3-DT||23.4µg/mL | | |experiment||Plac(SpeI+PstI)||11.6µg/mL||RBS-lysis3-DT||23.4µg/mL |
| Line 536: |
Line 536: |
| | |experiment||Plux(SpeI+PstI)||25µg/mL||RBS-lysis3-DT||23.4µg/mL | | |experiment||Plux(SpeI+PstI)||25µg/mL||RBS-lysis3-DT||23.4µg/mL |
| | |- | | |- |
| - | |experiment||Ptet-pT181 anti(SpeI+PstI)||30.6µg/mL||spin-DT(XbaI+PstI)||10.1µg/mL | + | |experiment||Ptet-pT181 anti(SpeI+PstI)||30.6µg/mL||spinach-DT(XbaI+PstI)||10.1µg/mL |
| | |- | | |- |
| | |experiment||Pcon-pT181 attenuator(SpeI+PstI)||14.5µg/mL||RBS-laczα-DT(XbaI+PstI)||4.4µg/mL | | |experiment||Pcon-pT181 attenuator(SpeI+PstI)||14.5µg/mL||RBS-laczα-DT(XbaI+PstI)||4.4µg/mL |
Revision as of 00:17, 27 September 2013
Sep 8
No name
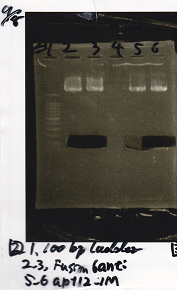
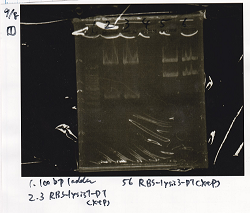
| Lane | DNA | Enzyme
|
| 1 | 100bp ladder |
|
| 2 | RBS-lysis1-DT | XbaI & PstI
|
| 3 | RBS-lysis1-DT | XbaI & PstI
|
| 5 | RBS-lysis3-DT | XbaI & PstI
|
| 6 | RBS-lysis3-DT | XbaI & PstI
|
| Name | concentration[µg/mL] | 260/280 | 260/230
|
| RBS-lysis1-DT (XbaI & PstI) | 5.3 | 1.98 | 0.06
|
| RBS-lysis3-DT (XbaI & PstI) | 5.3 | 1.98 | 0.06
|
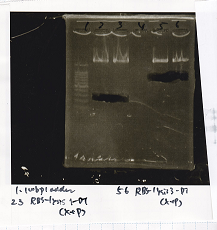
| Lane | DNA | Enzyme
|
| 1 | 100bp ladder |
|
| 3 | Pcon-pT181 attenuator | EcoRI & SpeI
|
| 4 | Pcon-pT181 attenuator | EcoRI & SpeI
|
| 6 | Spinach-DT | XbaI & PstI
|
| 7 | Spinach-DT | XbaI & PstI
|
| 9 | RBS-lacZα-DT | XbaI & PstI
|
| 10 | RBS-lacZα-DT | XbaI & PstI
|

| Name | concentration[µg/mL] | 260/280 | 260/230
|
| Pcon-pT181 attenuator (EcoRI & SpeI) | 5.2 | 1.82 | 0.36
|
| Spinach-DT (XbaI & PstI) | 8.0 | 1.87 | 0.25
|
| RBS-lacZα-DT (XbaI & PstI) | 10.3 | 1.71 | 0.54
|
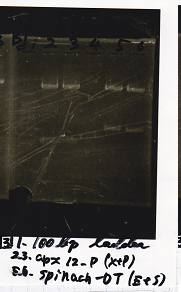
| Lane | DNA | Enzyme
|
| 1 | 1kbp ladder |
|
| 2 | Ptet-pT181 antisense | EcoRI & PstI
|
| 3 | Ptet-pT181 antisense | EcoRI & PstI
|
| 5 | pSB4K5 | EcoRI & SpeI
|
| 6 | pSB4K5 | EcoRI & SpeI
|


| Name | concentration[µg/mL] | 260/280 | 260/230
|
| Ptet-pT181 antisense(EcoRI & PstI) | 30.6 | 1.84 | 1.16
|
| pSB4K5 (EcoRI & SpeI) | 21.8 | 1.87 | 0.98
|
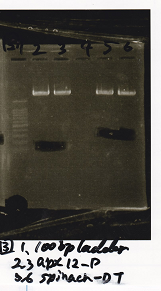
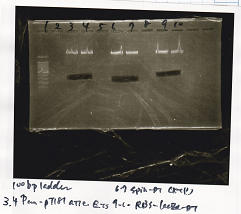
| Lane | DNA | Enzyme
|
| 1 | 1kbp ladder |
|
| 2 | Pcon-pT181 attenuator | SpeI & PstI
|
| 3 | Pcon-pT181 attenuator | SpeI & PstI
|
| 5 | Plac | SpeI & PstI
|
| 6 | Plac | SpeI & PstI
|
| 8 | pSB1C3 | EcoRI & SpeI
|
| 9 | pSB1C3 | EcoRI & SpeI
|
| 11 | pSB1C3 | XbaI & PstI
|
| 12 | pSB1C3 | XbaI & PstI
|


| Name | concentration[µg/mL] | 260/280 | 260/230
|
| Pcon-pT181 attenuator (SpeI & PstI) | 14.5 | 1.91 | 1.09
|
| Plac (SpeI & PstI) | 11.6 | 1.74 | 1.19
|
| pSB1C3 (EcoRI & SpeI) | 5.7 | 1.74 | 0.34
|
| pSB1C3 (XbaI & PstI) | 5.6 | 2.00 | 0.37
|
Miniprep
No name
| DNA | concentration[µg/mL] | 260/280 | 260/230
|
| Pcon-pT181 anti | 243.0 | 1.90 | 1.81
|
| Pcon-Spinach-DT | 253.7 | 1.57 | 1.51
|
Ligation
Kojima
| state | Vector(µL) | Inserter(µL) | Ligation High ver.2
|
| experiment | 9/8 Plac SpeI+PstI | 4.3 | 9/3 pT181 attenuator | 3.1 |
|
| experiment | 9/8 Plac SpeI+PstI | 4.3 | 9/7 aptamer12-1R-DT XbaI+PstI | 2.1 |
|
| experiment | 9/8 Plac SpeI+PstI | 4.3 | 9/8 spinach-DT XbaI+PstI | 4.6 |
|
| experiment | 9/6 Plac SpeI+PstI | 2 | 9/7 RBS-lysis2-DT XbaI+PstI | 10 |
|
| experiment | 9/8 Plac SpeI+PstI | 4.3 | 9/7 RBS-lysis2-DT XbaI+PstI | 10 |
|
| experiment | 9/8 Ptet-pT181 anti | 1.6 | 9/8 spinach-DT XbaI+PstI | 4.3 |
|
| experiment | 9/6 Pcon SpeI+PstI | 1.4 | 9/8 RBS-laczα-DT XbaI+PstI | 4.8 |
|
| experiment | 9/8 Pcon-pT181 attenuator SpeI+PstI | 3.4 | 9/7 aptamer12-1R-DT XbaI+PstI | 1.9 |
|
| experiment | 9/6 Pcon SpeI+PstI | 1.4 | 9/7 aptamer12-1R-DT XbaI+PstI | 2.1 |
|
| experiment | 9/8 DT | 3.0 | 9/8 Pcon-pT181 attenuator EcoRI+SpeI | 7.9 |
|
| experiment | 9/6 Plux SpeI+PstI | 2 | 9/7 RBS-lysis1-DT XbaI+PstI | 7.2 |
|
| experiment | 9/8 Plac SpeI+PstI | 4.3 | 9/7 RBS-lysis1-DT XbaI+PstI | 7.2 |
|
| experiment | 9/4 Plac SpeI+PstI | 1.7 | 9/8 RBS-laczα-DT XbaI+PstI | 4.8 |
|
Restriction Enzyme Digestion
Kojima
| | 9/8 Pcon-pT181 anti | EcoRI | SpeI | XbaI | PstI | BSA | Buffer | MilliQ | total
|
| 2 cuts | 8.2µL | 0µL | 1µL | 0µL | 1µL | 3µL | 3µL | 13.8µL | 30µL
|
| NC | 0.4µL | 0µL | 0µL | 0µL | 0µL | 1µL | 1µL | 7.6µL | 10µL
|
| | 9/8 Pcon-spinach-DT | EcoRI | SpeI | XbaI | PstI | BSA | Buffer | MilliQ | total
|
| 2 cuts | 7.9µL | 1µL | 1µL | 0µL | 0µL | 3µL | 3µL | 14.1µL | 30µL
|
| NC | 0.4µL | 0µL | 0µL | 0µL | 0µL | 1µL | 1µL | 7.6µL | 10µL
|
| | 8/21 F1 atte | EcoRI | SpeI | XbaI | PstI | BSA | Buffer | MilliQ | total
|
| 2 cuts | 6.5µL | 0µL | 0µL | 1µL | 1µL | 3µL | 3µL | 15.5µL | 30µL
|
| NC | 0.3µL | 0µL | 0µL | 0µL | 0µL | 1µL | 1µL | 7.7µL | 10µL
|
| | 8/21 F3m2 atte | EcoRI | SpeI | XbaI | PstI | BSA | Buffer | MilliQ | total
|
| 2 cuts | 6.0µL | 0µL | 0µL | 1µL | 1µL | 3µL | 3µL | 16µL | 30µL
|
| NC | 0.3µL | 0µL | 0µL | 0µL | 0µL | 1µL | 1µL | 7.7µL | 10µL
|
| | 8/21 F1 anti | EcoRI | SpeI | XbaI | PstI | BSA | Buffer | MilliQ | total
|
| 2 cuts | 6.7µL | 0µL | 0µL | 1µL | 1µL | 3µL | 3µL | 15.3µL | 30µL
|
| NC | 0.3µL | 0µL | 0µL | 0µL | 0µL | 1µL | 1µL | 7.7µL | 10µL
|
| | 8/21 F6 anti | EcoRI | SpeI | XbaI | PstI | BSA | Buffer | MilliQ | total
|
| 2 cuts | 6.0µL | 0µL | 0µL | 1µL | 1µL | 3µL | 3µL | 16µL | 30µL
|
| NC | 0.3µL | 0µL | 0µL | 0µL | 0µL | 1µL | 1µL | 7.7µL | 10µL
|
| | 8/21 tetR aptamer 12_1M | EcoRI | SpeI | XbaI | PstI | BSA | Buffer | MilliQ | total
|
| 2 cuts | 5.3µL | 1µL | 1µL | 0µL | 0µL | 3µL | 3µL | 16.7µL | 30µL
|
| NC | 0.3µL | 0µL | 0µL | 0µL | 0µL | 1µL | 1µL | 7.7µL | 10µL
|
| | 8/21 tetR aptamer 12_P | EcoRI | SpeI | XbaI | PstI | BSA | Buffer | MilliQ | total
|
| 2 cuts | 13.3µL | 1µL | 1µL | 0µL | 0µL | 3µL | 3µL | 8.7µL | 30µL
|
| NC | 0.7µL | 0µL | 0µL | 0µL | 0µL | 1µL | 1µL | 7.3µL | 10µL
|
| | 9/4 spinach-DT | EcoRI | SpeI | XbaI | PstI | BSA | Buffer | MilliQ | total
|
| 2 cuts | 5.4µL | 0µL | 0µL | 1µL | 1µL | 3µL | 3µL | 16.6µL | 30µL
|
| NC | 0.3µL | 0µL | 0µL | 0µL | 0µL | 1µL | 1µL | 7.7µL | 10µL
|
| | 9/7 RBS-lysis1-DT | EcoRI | SpeI | XbaI | PstI | BSA | Buffer | MilliQ | total
|
| 2 cuts | 10µL | 0µL | 0µL | 1µL | 1µL | 3µL | 3µL | 12µL | 30µL
|
| NC | 0.5µL | 0µL | 0µL | 0µL | 0µL | 1µL | 1µL | 7.5µL | 10µL
|
| | 9/6 RBS-lysis2-DT | EcoRI | SpeI | XbaI | PstI | BSA | Buffer | MilliQ | total
|
| 2 cuts | 13µL | 0µL | 0µL | 1µL | 1µL | 3µL | 3µL | 9µL | 30µL
|
| NC | 0.7µL | 0µL | 0µL | 0µL | 0µL | 1µL | 1µL | 7.3µL | 10µL
|
| | 9/7 RBS-lysis3-DT | EcoRI | SpeI | XbaI | PstI | BSA | Buffer | MilliQ | total
|
| 2 cuts | 10µL | 0µL | 0µL | 1µL | 1µL | 3µL | 3µL | 12µL | 30µL
|
| NC | 0.5µL | 0µL | 0µL | 0µL | 0µL | 1µL | 1µL | 7.5µL | 10µL
|
| | 8/20 J23100 RBS-lacZα-DT-(1) | EcoRI | SpeI | XbaI | PstI | BSA | Buffer | MilliQ | total
|
| 2 cuts | 7µL | 0.5µL | 0.5µL | 0µL | 0µL | 3µL | 3µL | 16µL | 30µL
|
| NC | 0.5µL | 0µL | 0µL | 0µL | 0µL | 1µL | 1µL | 7.5µL | 10µL
|
| | 8/20 RBS-lacZα-DT | EcoRI | SpeI | XbaI | PstI | BSA | Buffer | MilliQ | total
|
| 2 cuts | 10µL | 0.5µL | 0.5µL | 0µL | 0µL | 3µL | 3µL | 13µL | 30µL
|
| NC | 0.5µL | 0µL | 0µL | 0µL | 0µL | 1µL | 1µL | 7.5µL | 10µL
|
Transformation
Nakamoto & Ashida
| Name | Sample(µL) | Competent Cells(µL) | Total(µL) | Plate
|
| 9/8 Pcont+aptamer 12-1R-DT | 1 | 10 | 11 | Amp
|
| 9/8 Pcon+RBS-lacZα-DT | 1 | 10 | 11 | Amp
|
| 9/8 Pcon-pT181 attenuator+aptamer 12-1R-DT | 1 | 10 | 11 | Amp
|
| 9/8 Plac+RBS-lysis1-DT | 2 | 20 | 22 | CP
|
| 9/8 Plac+RBS-lysis2-DT | 2 | 20 | 22 | CP
|
| 9/8 Plac+aptamer 12-1R-DT | 2 | 20 | 22 | CP
|
| 9/8 Plac+spinach | 2 | 20 | 22 | CP
|
| 9/8 Plac+pT181 attenuator | 2 | 20 | 22 | CP
|
| 9/8 Plux+RBS-lysis1-DT | 1 | 10 | 11 | CP
|
| 9/8 Plux+RBS-lysis2-DT | 1 | 10 | 11 | CP
|
| 9/8 Ptet+RBS-lacZα-DT | 1 | 10 | 11 | CP
|
| 9/8 Ptet-pT181 anti+spinach-DT | 1 | 10 | 11 | CP
|
| 9/8 Pcon-pT181 attenuator+DT | 1 | 10 | 11 | CP
|
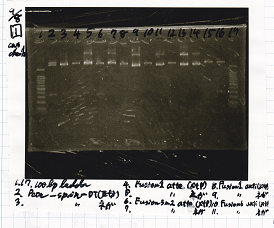
Electrophoresis
No name
| Lane | Sample | Enzyme1 | Enzyme2
|
| 1 | 100bp ladder | |
|
| 2 | Pcon-spinach-DT | EcoRI | SpeI
|
| 3 | Pcon-spinach-DT | |
|
| 4 | Fusion1 attenuator | XbaI | PstI
|
| 5 | Fusion1 attenuator | |
|
| 6 | Fusion3m2 attenuator | XbaI | PstI
|
| 7 | Fusion3m2 attenuator | |
|
| 8 | Fusion1 anti | XbaI | PstI
|
| 9 | Fusion1 anti | |
|
| 10 | Fusion6 anti | XbaI | PstI
|
| 11 | Fusion6 anti | |
|
| 12 | aptamer 12-1M | XbaI | PstI
|
| 13 | aptamer 12-1M | |
|
| 14 | aptamer 12-P | XbaI | PstI
|
| 15 | aptamer 12-P | |
|
| 16 | spinach-DT | XbaI | PstI
|
| Lane | Sample | Enzyme1 | Enzyme2
|
| 1 | spinach-DT | |
|
| 2 | J23100-RBS-lacZα-DT | EcoRI | SpeI
|
| 3 | J23100-RBS-lacZα-DT | |
|
| 4 | 100bp ladder | |
|
| 5 | RBS-lacZα-DT | EcoRI | SpeI
|
| 6 | RBS-lacZα-DT | |
|
| 7 | RBS-lysis1-DT | XbaI | PstI
|
| 8 | RBS-lysis1-DT | |
|
| Lane | Sample | Enzyme1 | Enzyme2
|
| 1 | 100bp ladder | |
|
| 2 | RBS-lysis2-DT | XbaI | PstI
|
| 3 | RBS-lysis2-DT | |
|
| 4 | RBS-lysis3-DT | XbaI | PstI
|
| 5 | RBS-lysis3-DT | |
|
| 6 | Pcon pT181 anti | SpeI | PstI
|
| 7 | Pcon pT181 anti | |
|

Ligation
No name
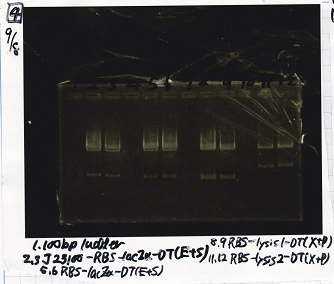
| state | Vector | Inserter
|
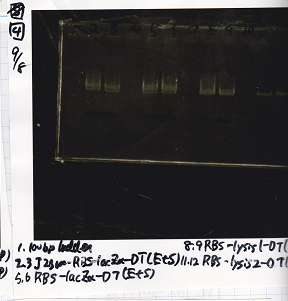
| experiment | Pcon-spinach-DT(EcoRI&SpeI) | 10.5µg/mL | pSB6A1(EcoRI&SpeI) | 8.9µg/mL
|
| experiment | Pcon-spinach-DT(EcoRI&SpeI) | 10.5µg/mL | pSB4K5(EcoRI&SpeI) | 21.8µg/mL
|
| experiment | Pcon-pT181 anti(SpeI+PstI) | 31.3µg/mL | spinach-DT(XbaI+PstI) | 10.1µg/mL
|
| experiment | Plac(SpeI+PstI) | 11.6µg/mL | RBS-lysis3-DT | 23.4µg/mL
|
| experiment | Plux(SpeI+PstI) | 25µg/mL | RBS-lysis3-DT | 23.4µg/mL
|
| experiment | Ptet-pT181 anti(SpeI+PstI) | 30.6µg/mL | spinach-DT(XbaI+PstI) | 10.1µg/mL
|
| experiment | Pcon-pT181 attenuator(SpeI+PstI) | 14.5µg/mL | RBS-laczα-DT(XbaI+PstI) | 4.4µg/mL
|
PCR
No name
| KaiA(plasmid) | KOD plus | 10x buffer | dNTP | MgSO4 | Primer(661-RBS-KaiA-fwd) | Primer(kaiA-662-rev-ver2) | MilliQ | total
|
| 1 | 0.5 | 2.5 | 2.5 | 1.5 | 0.75 | 0.75 | 15.5 | 25
|
| PreDenature | Denature | Annealing | Extension | cycle
|
| 94°C | 98°C | 57°C | 68°C | --
|
| 2min | 10s | 30s | 28s | 30cycles
|
| KaiB(plasmid) | KOD plus | 10x buffer | dNTP | MgSO4 | Primer(662-RBS-KaiB-fwd-ver2) | Primer(kaiB-663-rev-ver2) | MilliQ | total
|
| 1 | 0.5 | 2.5 | 2.5 | 1.5 | 0.75 | 0.75 | 15.5 | 25
|
| PreDenature | Denature | Annealing | Extension | cycle
|
| 94°C | 98°C | 57°C | 68°C | --
|
| 2min | 10s | 30s | 28s | 30cycles
|
| DT(plasmid) | KOD plus | 10x buffer | dNTP | MgSO4 | Primer(664-DT-fwd-ver2) | Primer(DT-suffix-660-rev-ver2) | MilliQ | total
|
| 0.1 | 0.5 | 2.5 | 2.5 | 1.5 | 0.75 | 0.75 | 16.4 | 25
|
| PreDenature | Denature | Annealing | Extension | cycle
|
| 94°C | 98°C | 57°C | 68°C | --
|
| 2min | 10s | 30s | 28s | 30cycles
|
| P-KaiB(PCR prpduct) | KOD plus | 10x buffer | dNTP | MgSO4 | Primer(prefix-PkaiBC-fwd-ver2) | Primer(PkaiBC-suffix--662-rev-ver2) | MilliQ | total
|
| 0.7 | 0.5 | 2.5 | 2.5 | 1.5 | 0.75 | 0.75 | 15.8 | 25
|
| PreDenature | Denature | Annealing | Extension | cycle
|
| 94°C | 98°C | 57°C | 68°C | --
|
| 2min | 10s | 30s | 28s | 30cycles
|
| Plac(1)(plasmid) | KOD plus | 10x buffer | dNTP | MgSO4 | Primer(GG0-pSBIC3-fwd-ver2) | Primer(Plac-GG1-rev-ver2) | MilliQ | total
|
| 0.13 | 0.5 | 2.5 | 2.5 | 1.5 | 0.75 | 0.75 | 16.37 | 25
|
| PreDenature | Denature | Annealing | Extension | cycle
|
| 94°C | 98°C | 57°C | 68°C | --
|
| 2min | 10s | 30s | 60s | 30cycles
|
| KaiC(plasmid) | KOD plus | 10x buffer | dNTP | MgSO4 | Primer(GG3-RBS-KaiC-fwd-ver2) | Primer(KaiC-GG4-rev-ver2) | MilliQ | total
|
| 1 | 0.5 | 2.5 | 2.5 | 1.5 | 0.75 | 0.75 | 15.5 | 25
|
| PreDenature | Denature | Annealing | Extension | cycle
|
| 94°C | 98°C | 57°C | 68°C | --
|
| 2min | 10s | 30s | 60s | 30cycles
|
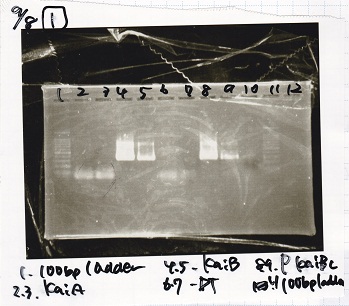
Yoshida
| Lane | DNA
|
| 1 | 100bp ladder
|
| 2 | KaiA
|
| 3 | KaiA
|
| 4 | KaiB
|
| 5 | KaiB
|
| 6 | DT
|
| 7 | DT
|
| 8 | P-KaiBC
|
| 9 | P-KaiBC
|
| 10 | nega
|
| 11 | 100bp ladder
|

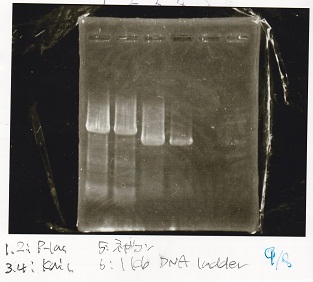
| Lane | DNA
|
| 1 | Plac
|
| 2 | Plac
|
| 3 | KaiC
|
| 4 | KaiC
|
| 5 | nega
|
| 6 | 1kb ladder
|

 "
"