Team:UNIK Copenhagen/Signe Notebook
From 2013.igem.org
Emilfischer (Talk | contribs) |
Emilfischer (Talk | contribs) |
||
| Line 268: | Line 268: | ||
<u>Assembly of constructs</u> | <u>Assembly of constructs</u> | ||
<br> | <br> | ||
| - | Colonies 1, 2, 3 (MamC-pJET) and II, IV, VIII (eGFP-pBBR1MCS-2) were setup in liquid cultures (see colony PCR from previous week). Miniprep was carried out and plasmids were sent for sequencing. All MamC sequences were perfect while all eGFP sequences were wrong. The cloning of eGFP into pDRIVE was successfully redone (documentation lost). Fluorescence was also measured of | + | Colonies 1, 2, 3 (MamC-pJET) and II, IV, VIII (eGFP-pBBR1MCS-2) were setup in liquid cultures (see colony PCR from previous week). Miniprep was carried out and plasmids were sent for sequencing. All MamC sequences were perfect while all eGFP sequences were wrong. The cloning of eGFP into pDRIVE was successfully redone (documentation lost). Fluorescence was also measured of eFbFP-pBBR1MCS-2 strains. No fluorescence detected as the sequencing explains. Inducing with IPTG (final conc. 1 mM) had no effect. |
<br><br> | <br><br> | ||
| Line 285: | Line 285: | ||
<div class="left_page"> | <div class="left_page"> | ||
<h1>Week 6</h1> | <h1>Week 6</h1> | ||
| - | <p> | + | <p> |
| + | |||
| + | <u>Culturing of MTBs</u> | ||
| + | <u>Assembly of constructs</u> | ||
| + | |||
| + | |||
| + | |||
| + | </p> | ||
</div> | </div> | ||
<div class="right_page"> | <div class="right_page"> | ||
Revision as of 06:22, 22 September 2013
Notebook
Get our Nootebook in a printable version here .
Week 1 - July 8th-14th
Culturing of magnetotactic bacteria (MTBs)
Making base medium for growing magnetotactic bacteria. This medium was also used to make agar plates.
MS-1 inoculated in liquid base medium. Falcon tubes were flushed with the nitrogen before use and incubated at 28 degrees.
Plates were kept in a closed container with an Oxoid AnaeroGen pad.
Assembly of constructs
Glycerol stock of E. coli with pBBR1MCS-2 was thawed to grow on these plates.
Primers for MamC (MS-1 strain) was ordered.
Week 3
Culturing of MTBs
making charcoal media and plates. MS-1 and MSR-1 was inoculated. Liquid cultures were setup in Hungate tubes 200ul, 400ul and 600ul of each strain (MS-1 and MSR-1). Charcoal plates were inoculated as well with both strains.
Assembly of constructs
Colony PCRs of eFbFP-pEX-A and eGFP-pDRIVE. Only eFbFP was successful.

Overnight cultures were made of colonies 1-4 (eFbFP-pEX-A). Miniprep of eFbFP-pEX-A
cultures. Samples sent for sequencing.
Overnight cultures were made of colonies 1-4 (eFbFP-pEX-A).
Miniprep of eFbFP-pEX-A cultures. Samples sent for sequencing.
Colony PCR of eGFP-pDRIVE was repeated. Still negative.
Cloning and transformation was also repeated. CloneJET PCR cloning kit was used to create eGFP-pJET (pJET was chosen instead of pDRIVE). Not successful.
Gradient PCR of eGFP premade vector (56-65 degrees). Gel electrophoresis was carried out and the bands were purified from the gel.
An attempt to clone eGFP into pDRIVE was not successful.
The cloning of eFbFP into expression vector pBBR1MCS-2 was successful instead.
Colony PCR of eGFP-pDRIVE and eFbFP-pBBR1MCS-2. Gel electrophoresis revealed only one colony to be successful (eFbFP-pBBR1MCS-2 no. 17).
Week 4
Assembly of constructs
Colony PCR of eGFP-pDRIVE was repeated with increased elongation time (1 min.)
 Cloning looks successful and colonies 1-4 were set up as liquid cultures overnight.
Cloning looks successful and colonies 1-4 were set up as liquid cultures overnight.
Colony PCR of eFbFP-pBBR1MCS-2 because only one colony was positive in week 3. Increased elongation time.
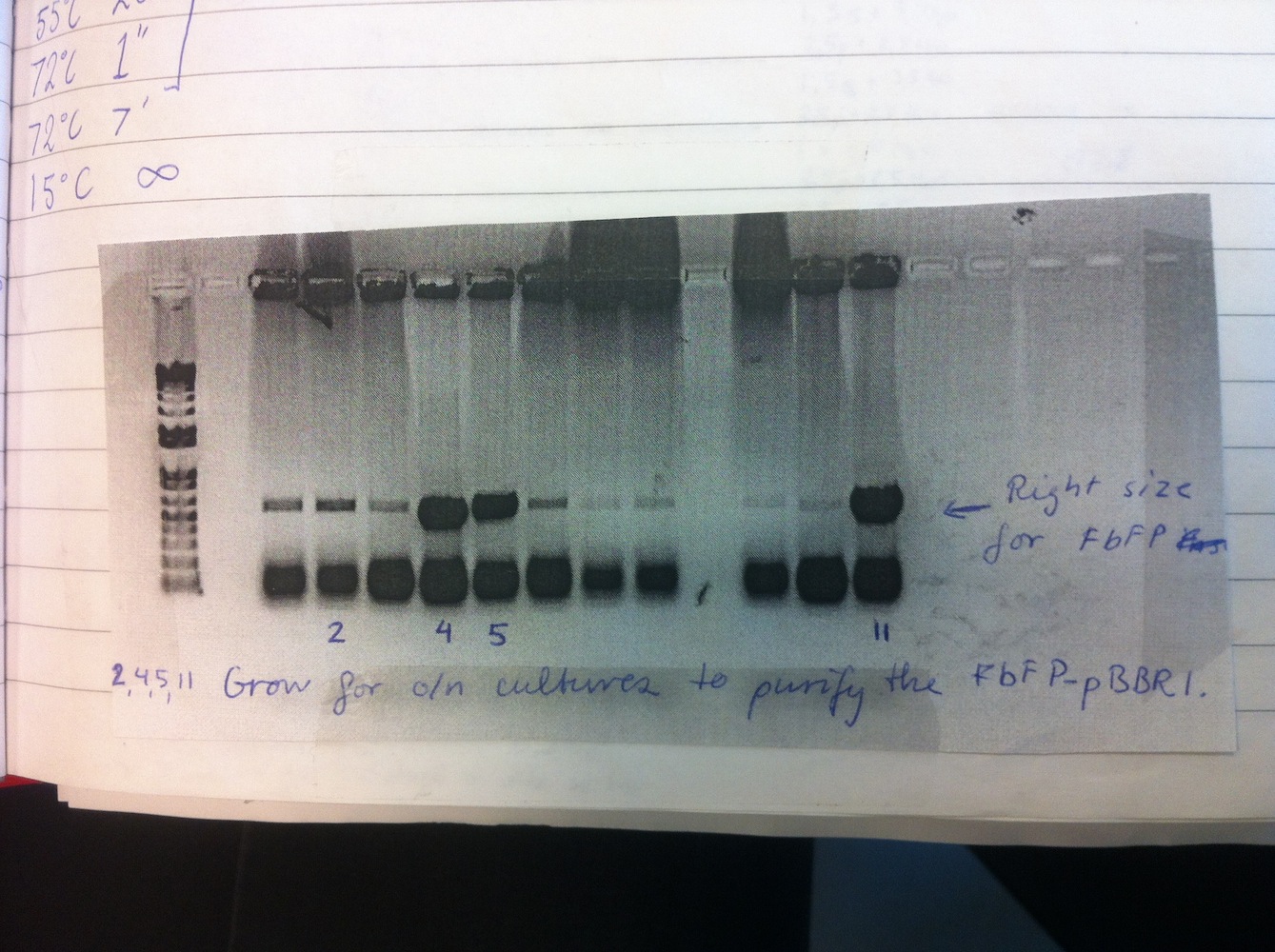
Overnight liquid cultures were setup for colonies 2, 4, 5 and 11.
Miniprep was performed on these cultures alongside eGFP-pDRIVE colonies 1-4. This gave good yields and the plasmids were sent for sequencing. This showed eGFP-pDRIVE no. 4 and eFbFP-pBBR1MCS-2 no. 17 was correct!
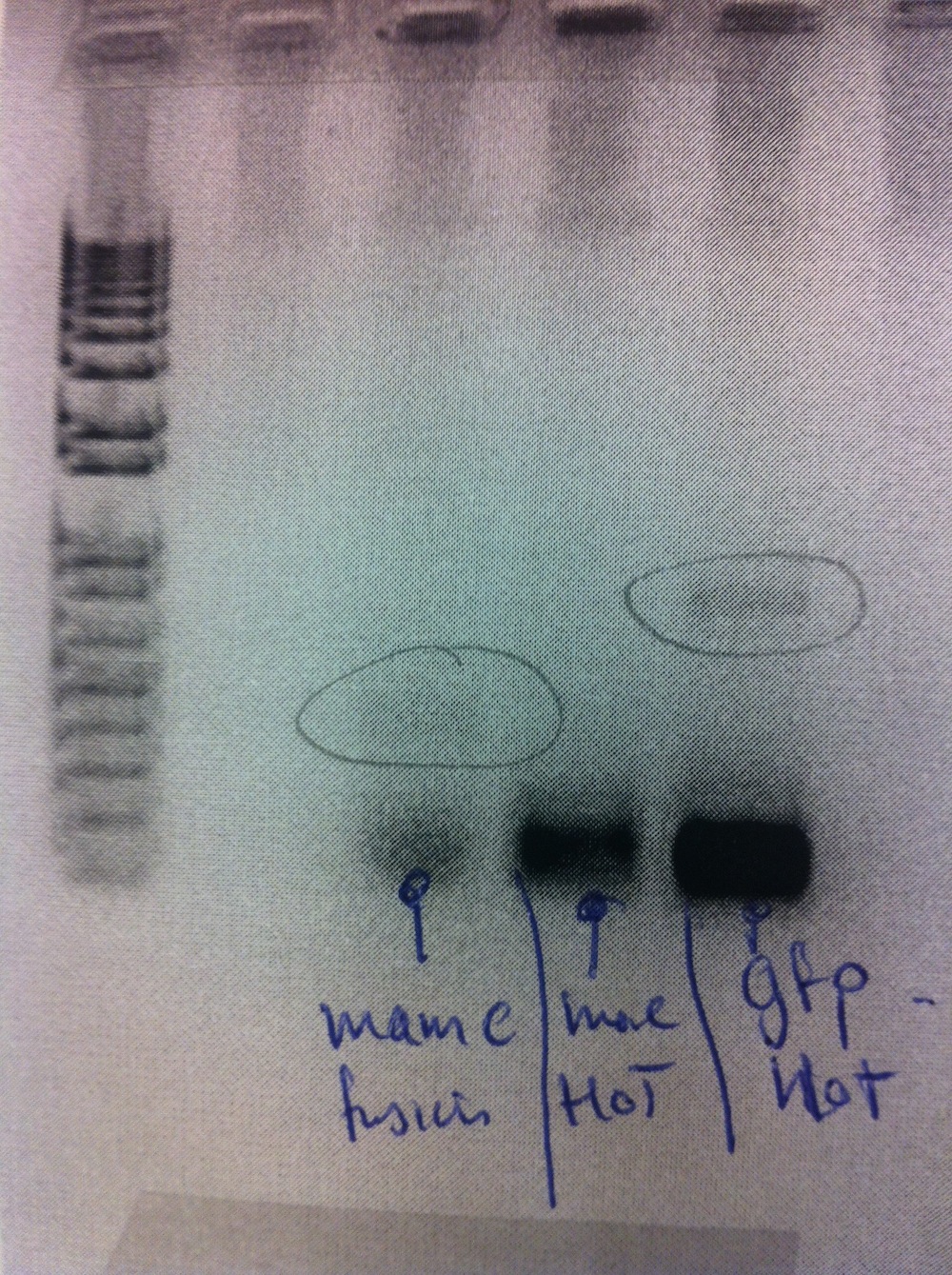
Cloning and transformation of eGFP-pBBR1MCS-2 using plasmid no. 4 (mentioned just above). The transformation only gave rise to 2 colonies, thus suggesting the cloning to be unsuccessful. The cloning was repeated and transformation was carried out again. Colony PCR was performed alongside MamC-pJET (see gel below).
Liquid cultures were setup from eFbFP-pBBR1MCS-2 no. 2, 4, 5, 11, 17 for 2 hours in order to measure fluorescence. First, OD(600nm) was measured to ensure equal density of cells. The measurements did not show fluorescence (ex. 450 nm, em. 495 nm) from the cultures.
Cloning and transformation of MamC-pDRIVE. Colony PCR showed the cloning to be of cryptic success. Colony PCR was redone without success.
Cloning was repeated using the CloneJET PCR cloning kit. MamC-pJET was transformed and plated. Colony PCR was carried out alongside eGFP-pBBR1MCS-2.
 The gel electrophoresis indicated MamC-pJET to be successfully cloned. However, only a few of the eGFP-pBBR1MCS-2 colonies appeared to have contain the insert.
The gel electrophoresis indicated MamC-pJET to be successfully cloned. However, only a few of the eGFP-pBBR1MCS-2 colonies appeared to have contain the insert.
Week 5
Culturing of MTBs
Colony PCR using MamC primer was done with MS-1 and MSR-1 colonies. MamC was amplified from the genomic DNA. This did not give rise to any bands via gel electrophoresis.
MamC was, however, successfully amplified from MSR-1 later in the week (see gel below).

Assembly of constructs
Colonies 1, 2, 3 (MamC-pJET) and II, IV, VIII (eGFP-pBBR1MCS-2) were setup in liquid cultures (see colony PCR from previous week). Miniprep was carried out and plasmids were sent for sequencing. All MamC sequences were perfect while all eGFP sequences were wrong. The cloning of eGFP into pDRIVE was successfully redone (documentation lost). Fluorescence was also measured of eFbFP-pBBR1MCS-2 strains. No fluorescence detected as the sequencing explains. Inducing with IPTG (final conc. 1 mM) had no effect.
The CPH strain
Enrichment of magnetotactic bacteria was done according to the protocol. It was unclear whether the bacteria observed in the microscope were actually magnetotactic. They were compared to MS-1 and MSR-1 samples.
Week 6
Culturing of MTBs Assembly of constructs
more text on this page!
Week 7
This week the team...
more text on this page!
Week 8
This week the team...
more text on this page!
Week 9
This week the team...
more text on this page!
Week 10
This week the team...
more text on this page!
Week 11
This week the team...
more text on this page!
Week 12
This week the team...
more text on this page!
 "
"