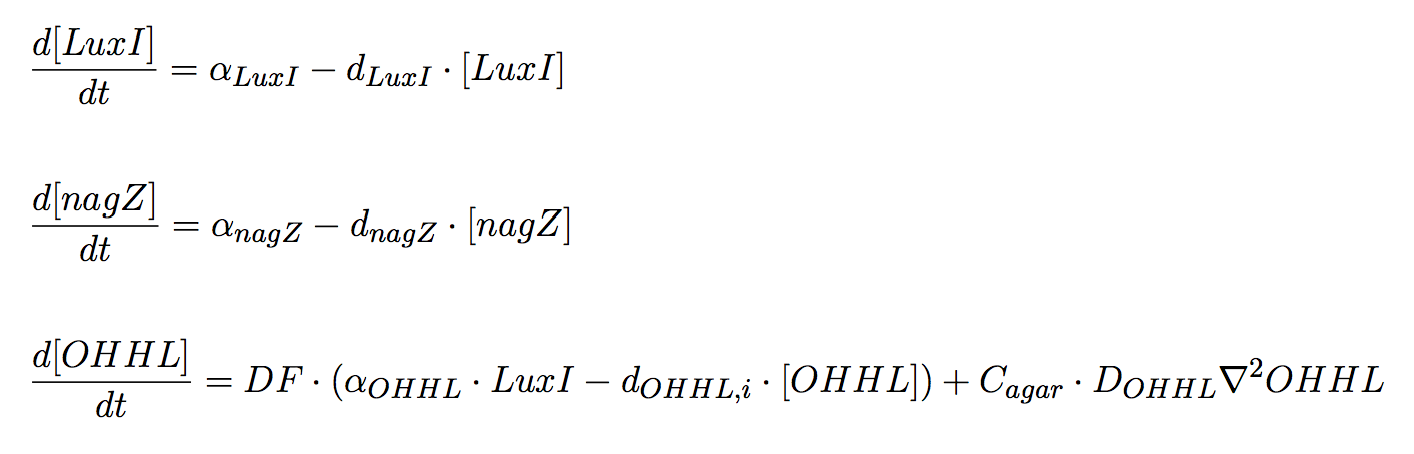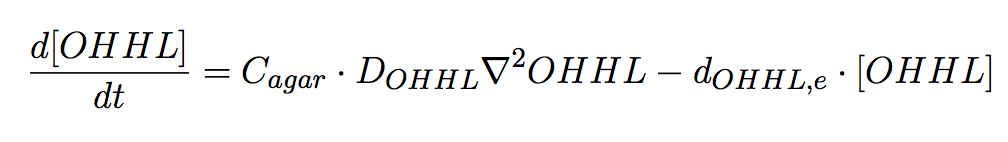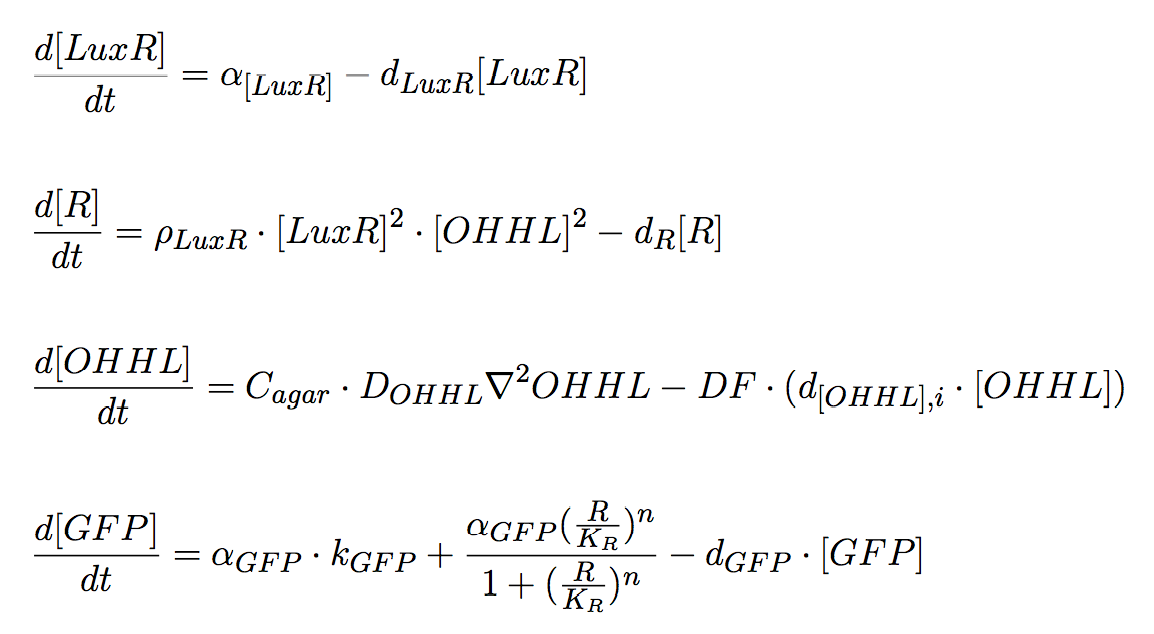Team:ETH Zurich/GFP
From 2013.igem.org
| Line 60: | Line 60: | ||
<h1>Results: Simulation</h1> | <h1>Results: Simulation</h1> | ||
| - | <p align="justify"> The simulation was conducted for 11 hours, with 3 mine cells following the experimental setup (locations: row 2-third colony, row 3-fifth colony, row 4 | + | <p align="justify"> The simulation was conducted for 11 hours, with 3 mine cells following the experimental setup (locations: row 2 - third colony, row 3 - fifth colony, row 4 - fourth colony). From the simulation, one can see that the predicted time scale for the expression of GFP and the experimental observations are congruent. In addition, another two observations from these results are very important: </p><br> |
: 1. A proper OHHL gradient can be formed to trigger expression of a protein under the control of a promoter activated by the dimer LuxR/OOHL. In this case, we can observed that the intensity of GFP correlates with the number of neighbours in the immediate vicinity.<br> | : 1. A proper OHHL gradient can be formed to trigger expression of a protein under the control of a promoter activated by the dimer LuxR/OOHL. In this case, we can observed that the intensity of GFP correlates with the number of neighbours in the immediate vicinity.<br> | ||
: 2. The contribution from the basal expression of protein cannot be neglected. <br><br> | : 2. The contribution from the basal expression of protein cannot be neglected. <br><br> | ||
Revision as of 10:26, 25 September 2013
Contents |
Reaction-Diffusion Model: genetic circuit with GFP as reporter gene
In our first spatio-temporal model, we wanted to find out if (i) a suitable AHL gradient forms at all and (ii) validate the model with experimental data. Essentially we model the receiver cells (E. coli DH5α strain) being transformed with a plasmid containing GFP under the control of pLux promoter. Subsequently, we simulate a 2D spatio-temporal reaction-diffussion system with COMSOL Multiphysics.
AHL: Reaction-Diffusion Equation
The change of AHL concentration over time is influenced by two processes: (i) local chemical reactions and (ii) diffusion; which causes the molecule to spread over the agar plate (Eq. 1).
For diffusion, we have a partial differential equation (Eq. 2) which describes density fluctuations over time and space. We do not model OOHL diffusion in and out cells explicitly; the underlying assumption is that this process is fast or that the molecule freely diffuses. From equation 2, DOOHL(OOHL(r,t),r) denotes the collective diffusion coefficient for OOHL at location r. However, we assume that the diffusion coefficient does not depend on the density, i.e., DOOHL is a constant. The value reported in the literature for the diffusion constant corresponds to measurements performed in water at 25oC. Since diffusion in our system happens in agar, we scaled the diffusion constant by a factor Cagar (Fatin-Rouge et al., 2004).
For the reaction component, the change of AHL concentrations over time is given by an ordinary differential equation (ODE), that comprises production and linear degradation. The synthesis of the signalling molecule depends on the product of luxI gene. Now for the degradation, we consider that AHL degrades at different rates depending on the localization, i.e. cytoplasmic or extracellular. Given that the intracellular degradation is driven by enzymatic degradation, whereas the extracellular decay is a non active process. Our model also includes a dilution factor due to the cell growth (Eq. 3).
Finally, we need to specify the initial conditions (at time t = 0) and boundary conditions. At the starting point there is no OOHL in the agar plate, thus the initial concentration is zero ([OOHL(r,t=0)] = 0 M). For the boundary condition, we take into account that there is not flux out of the agar plate.
ODE's for other species
OOHL is the only molecule in the model that is allowed to diffuse in and out cells, hence the change of concentrations over time for the other species is given by system of non-linear ordinary differential equations (ODEs). Each equation represents the rate of change of species' continuos concentration as a sum of terms representing biological processes, such as production, degradation and regulation; the later is captured with Michaelis-Menten functions. An important assumption is that specific cellular processes like transcription and translation are not modelled explicitly, but lumped together.
Our system consists of two type of genetically engineered colonies: (i) Mine Cells and (ii) Receiver Cells, that can be treated as modules that communicate only by a single molecule, OOHL.
Mine Cells
Two proteins of interest for our design are produced by mine cells: (i) LuxI and (ii) NagZ; both genes are constitutively expressed. Aditionally, the synthesis of AHL is carried on by mine cells; specifically, the product of luxI gene is directly involved in the synthesis of the molecule, using as substrates S-adenosylmethionine (SAM) and an acylated acyl carrier protein (ACP) from the fatty acid biosynthesis pathway (Schaefer et al., 1996). We assume precursor molecules are provided by the cell in non-limiting conditions.
The PDEs for the states involved in the sender module are given below:
Agar Plate
In the agar plate no reactions take place; hence only two processes of interested are considered: (i) diffusion and (i))decay of the diffusible AHL signal (Eq 6).
Receiver Cells
Receiver Cells are responsible for processing the signal sent by the mine cells. The input is the signalling molecule, AHL, and the output is a fluorescence signal whose intensity correlates with the sensed AHL concentration; for this purpose GFP expression is under control of pLux promoter. Once AHL molecules reach a non-mine colony, it can bind to LuxR protein, and the complex LuxR/AHL acts as a transcriptional activator. Therefore, this module works as a high pass amplitude filter for GFP, since it is produced when the AHL levels are sufficiently high.
The PDEs for the states involved in the sender module are given below:
Results: Simulation
The simulation was conducted for 11 hours, with 3 mine cells following the experimental setup (locations: row 2 - third colony, row 3 - fifth colony, row 4 - fourth colony). From the simulation, one can see that the predicted time scale for the expression of GFP and the experimental observations are congruent. In addition, another two observations from these results are very important:
- 1. A proper OHHL gradient can be formed to trigger expression of a protein under the control of a promoter activated by the dimer LuxR/OOHL. In this case, we can observed that the intensity of GFP correlates with the number of neighbours in the immediate vicinity.
- 2. The contribution from the basal expression of protein cannot be neglected.
 "
"