Team:Goettingen/Team/DAC
From 2013.igem.org
(→Introduction) |
(→Results and Discussion) |
||
| Line 51: | Line 51: | ||
<html><img src="https://static.igem.org/mediawiki/2013/5/51/Goe-greenColi-labcoat.png" style="height: 200px;position: absolute;top: 1347px;left: 361px;" /></html> | <html><img src="https://static.igem.org/mediawiki/2013/5/51/Goe-greenColi-labcoat.png" style="height: 200px;position: absolute;top: 1347px;left: 361px;" /></html> | ||
<html><img src="https://static.igem.org/mediawiki/2013/6/6e/Goe-greenColi-crystal.png" style="position: absolute;top: 3565px;left: 500px;z-index: 3;" /></html> | <html><img src="https://static.igem.org/mediawiki/2013/6/6e/Goe-greenColi-crystal.png" style="position: absolute;top: 3565px;left: 500px;z-index: 3;" /></html> | ||
| - | As the cloning of the full-length (273 aa) membrane-bound DacA (Lmo2120) into | + | As the cloning of the full-length (273 aa) membrane-bound DacA (Lmo2120) into Escherichia coli failed, we excluded the trans-membrane domains, meaning that we chopped off the first 100 amino acids. Nevertheless, the resulting truncated part still included the essential cyclase domain, and therefore represents one of our favorite BioBricks: [http://parts.igem.org/Part:BBa_K1045003 BBa_K1045003]! |
| - | + | By conducting several experiments, we proved that the truncated DacA protein (BBa_K1045003) was not only active ''in vivo'', but also ''in vitro''. Moreover, we were able to purify the diadenylate cyclase in large scale for determining its 3D structure! Using the structure data, one can now search for chemical compounds that interfere with the activity of the cyclase either by testing with available chemical libraries or by computational modeling. In the following text, the experiments will be explained in more detail. However, if you wish to get even more details, please visit the [[Team:Goettingen/Parts|Parts Registry]] or [[Team:Goettingen/NoteBook|our LabBook.]] | |
| - | + | The truncated DacA protein ([http://parts.igem.org/Part:BBa_K1045003 BBa_K1045003]) was extended with an N-terminal Step-tag allowing easy purification steps. The expression of our protein was brought under the control of a T7-promoter, enabling us to induce the expression by addition of Isopropyl-β-D-thiogalactopyranoside (IPTG). The plasmid was then cloned into the ''E. coli'' strain BL21, which is a protein expression strain. The Gram-negative bacterium ''E. coli'' does not produce c-di-AMP and is not severely affected by the signaling molecule in contrast to Gram-positive bacteria. | |
| - | + | ||
| - | + | ||
| - | The truncated DacA protein ([http://parts.igem.org/Part:BBa_K1045003 BBa_K1045003]) was extended with an N-terminal | + | |
In order to analyze the cyclase activity ''in vivo'', the ''E. coli'' clones were induced to express the protein by the addition of IPTG. The cells were then lysed to extract c-di-AMP from the cells. | In order to analyze the cyclase activity ''in vivo'', the ''E. coli'' clones were induced to express the protein by the addition of IPTG. The cells were then lysed to extract c-di-AMP from the cells. | ||
| - | By performing SDS gel electrophoresis it was nicely shown that the desired protein was highly expressed (Fig. 1). Furthermore, the presence of c-di-AMP in the supernatant of the lysed bacteria was confirmed using LC-MS/MS. Thus, one can conclude that our truncated DacA protein codes for an active adenylate cyclase domain ''in vivo''. | + | By performing the SDS gel electrophoresis it was nicely shown that the desired protein was highly expressed (Fig. 1). Furthermore, the presence of c-di-AMP in the supernatant of the lysed bacteria was confirmed using LC-MS/MS. Thus, one can conclude that our truncated DacA protein codes for an active adenylate cyclase domain ''in vivo''. |
https://static.igem.org/mediawiki/2013/7/70/Goe-dac-fig-1.png | https://static.igem.org/mediawiki/2013/7/70/Goe-dac-fig-1.png | ||
| Line 69: | Line 66: | ||
| - | Diadenylate cyclases catalyze the condensation reaction of two molecules ATP to a single molecule c-di-AMP while releasing two pyrophosphate molecules consisting of the β-γ-phosphates of each ATP (Fig. 2). In order to analyze the cyclase activity of DacA | + | Diadenylate cyclases catalyze the condensation reaction of two molecules of ATP to a single molecule c-di-AMP, while releasing two pyrophosphate molecules consisting of the β-γ-phosphates of each ATP (Fig. 2). In order to analyze the cyclase activity of DacA in vitro, we performed an assay in which these pyrophosphate molecules were used as evidence for the reaction. Among other things, the assay included a pyrophosphatase that cleaves the pyrophosphate molecule into free phosphate molecules, malachite-green and molybdate. Malachite-green forms a complex with free phosphate molecules and molybdate stabilizes the complex. The product absorption can be measured at 630 nm. With this ''in vitro'' assay, the concentration of free phosphate molecules and thus the conversion rate of ATP to c-di-AMP was analyzed. |
| - | Our results confirmed that DacA Lmo2120 acts as an active enzyme in the presence of a divalent cation, ATP and a buffer system at pH 8 (''in vitro''), however, the catalysis rate ''in vivo'' | + | Our results confirmed that DacA Lmo2120 acts as an active enzyme in the presence of a divalent cation, ATP and a buffer system at pH 8 (''in vitro''), however, the catalysis rate ''in vivo'' appears to be much more efficient. |
<html><a href="https://static.igem.org/mediawiki/2013/7/7f/Goe-dac-fig-2.png" target="_blank"><img src="https://static.igem.org/mediawiki/2013/7/7f/Goe-dac-fig-2.png" width="750"/></a></html> | <html><a href="https://static.igem.org/mediawiki/2013/7/7f/Goe-dac-fig-2.png" target="_blank"><img src="https://static.igem.org/mediawiki/2013/7/7f/Goe-dac-fig-2.png" width="750"/></a></html> | ||
| Line 78: | Line 75: | ||
| - | Finally, we are coming to the core of our project, the protein structure of DacA! The molecular structure of DacA can lead to the discovery of new antibacterial substance classes | + | Finally, we are coming to the core of our project, the protein structure of DacA! The molecular structure of DacA is very beneficial to have as it makes ''in silico'' experiments possible, which can lead to the discovery of new antibacterial substance classes that later can be used to treat patients. |
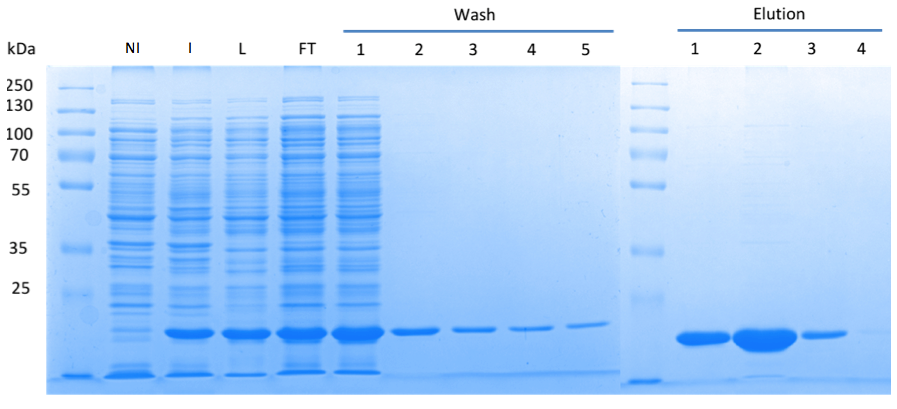
| - | In order to purify a large amount of this protein, our BioBrick [http://partsregistry.org/Part:BBa_K1045003 BBa_K1045003] with an N-terminal Strep-tag was expressed in ''E. coli'' under the control of a T7-promoter. The expression was again induced by supplementing the growth medium with IPTG. The high expression was confirmed by SDS gel electrophoresis with a resulting thick band at the size of 20 kDa (Fig. 3). The ''E. coli'' strain was grown in large scale | + | In order to purify a large amount of this protein, our BioBrick [http://partsregistry.org/Part:BBa_K1045003 BBa_K1045003] with an N-terminal Strep-tag was expressed in ''E. coli'' under the control of a T7-promoter. The expression was again induced by supplementing the growth medium with IPTG. The high expression was confirmed by SDS gel electrophoresis with a resulting thick band at the size of approximately 20 kDa (Fig. 3). The ''E. coli'' strain was grown in large scale (10 liters), the cells were pelletized, lysed and the protein was purified (Fig. 3). |
<html><a href="https://static.igem.org/mediawiki/2013/f/fe/Goe-dac-fig-3.png" target="_blank"><img src="https://static.igem.org/mediawiki/2013/f/fe/Goe-dac-fig-3.png" width="750" /></a></html> | <html><a href="https://static.igem.org/mediawiki/2013/f/fe/Goe-dac-fig-3.png" target="_blank"><img src="https://static.igem.org/mediawiki/2013/f/fe/Goe-dac-fig-3.png" width="750" /></a></html> | ||
| Line 86: | Line 83: | ||
Fig. 3. '''SDS gel showing overexpression and purification of DacA.''' (A) Lane 1: Thermo Scientific PageRuler Plus Prestained Protein Ladder; NI: Non-induced, Clone contained the cyclase domain of which the activity was not induced; I: Induced, Clone contained the cyclase domain of which the activity was induced by addition of isopropyl-ß-D-1-thiogalactopyranoside (IPTG); L: Lysate; FT: Flow-through; After wash 1 our protein was purified. | Fig. 3. '''SDS gel showing overexpression and purification of DacA.''' (A) Lane 1: Thermo Scientific PageRuler Plus Prestained Protein Ladder; NI: Non-induced, Clone contained the cyclase domain of which the activity was not induced; I: Induced, Clone contained the cyclase domain of which the activity was induced by addition of isopropyl-ß-D-1-thiogalactopyranoside (IPTG); L: Lysate; FT: Flow-through; After wash 1 our protein was purified. | ||
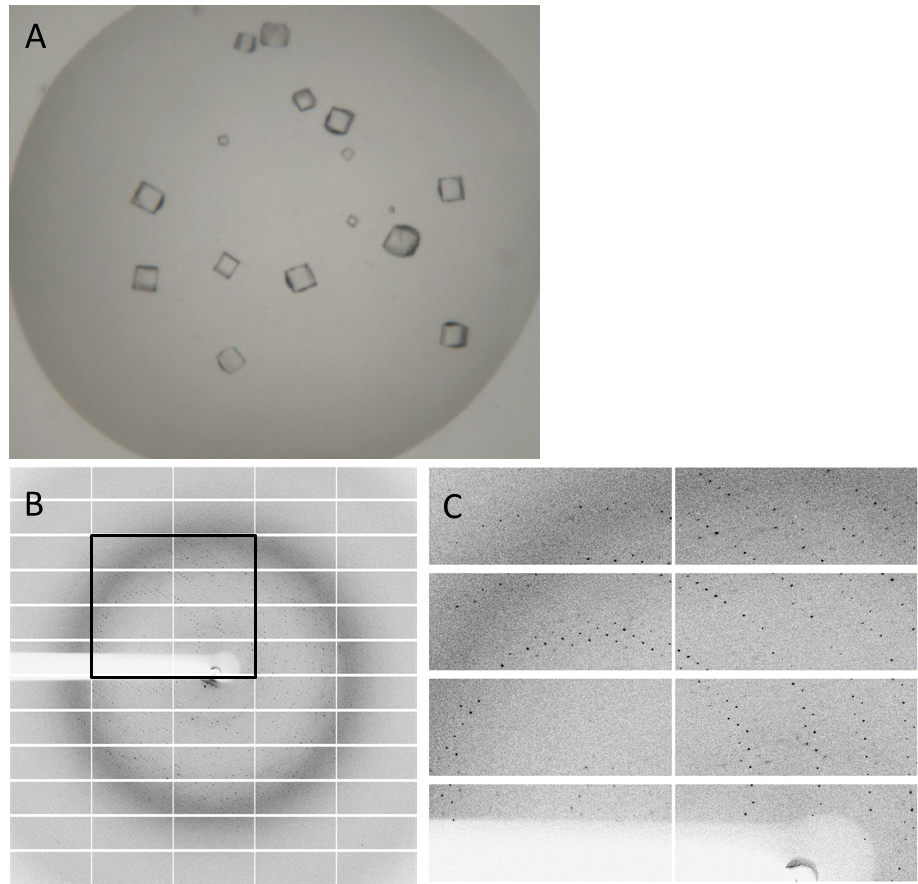
| - | Having dialyzed and concentrated the eluted protein, we obtained a protein concentration of 10 mg/ml, which was sufficient to perform the crystallization reaction. Very nice crystals were | + | Having dialyzed and concentrated the eluted protein, we obtained a protein concentration of about 10 mg/ml, which was sufficient to perform the crystallization reaction. Very nice crystals were obtained during trials when testing with different conditions (Fig. 4A). In order to find the perfect supplements for growing our crystals, the whole procedure was repeated. The crystals yielded an x-ray diffraction pattern, with a resolution of 2,8 Å (Fig. 4B,C). The dataset was measured at the EMBL Hamburg Beamline P13 at the PETRA III synchrotron on the DESY campus. |
| - | Finally, we were also able to obtain the protein structure (Fig. 5)! The structure shows a globular protein with a distinct ATP-binding cleft. The ribbon model demonstrates the | + | Finally, we were also able to obtain the protein structure (Fig. 5)! The structure shows a globular protein with a distinct ATP-binding cleft. The ribbon model demonstrates the secondary structure composed of α-helices and β-sheets. |
<html><a href="https://static.igem.org/mediawiki/2013/a/a8/Goe-dac-fig-4.png" target="_blank"><img src="https://static.igem.org/mediawiki/2013/a/a8/Goe-dac-fig-4.png" width="750"></a></html> | <html><a href="https://static.igem.org/mediawiki/2013/a/a8/Goe-dac-fig-4.png" target="_blank"><img src="https://static.igem.org/mediawiki/2013/a/a8/Goe-dac-fig-4.png" width="750"></a></html> | ||
Revision as of 19:00, 4 October 2013
 "
"