Team:Bielefeld-Germany/Modelling/Optimal
From 2013.igem.org
m |
|||
| (9 intermediate revisions not shown) | |||
| Line 2: | Line 2: | ||
{{Team:Bielefeld-Germany/css/header_cleanup.css}} | {{Team:Bielefeld-Germany/css/header_cleanup.css}} | ||
{{Team:Bielefeld-Germany/css/button.css}} | {{Team:Bielefeld-Germany/css/button.css}} | ||
| + | |||
| + | |||
__NOTOC__ | __NOTOC__ | ||
| Line 7: | Line 9: | ||
<html> | <html> | ||
<style> | <style> | ||
| + | h1{margin-top:70px; } | ||
#globalwrapper ul {padding-left:40px; padding-right:40px;} | #globalwrapper ul {padding-left:40px; padding-right:40px;} | ||
| Line 12: | Line 15: | ||
h2,h3,h4{clear:both;} | h2,h3,h4{clear:both;} | ||
| - | # | + | #leftcol h4{color:#ff6600; padding-left:20px;} |
| - | # | + | #leftcol div.thumb.tleft{margin-left:20px; margin-right:20px; clear:both;} |
| - | # | + | #leftcol ul {clear:both;} |
| - | # | + | #leftcol ul ul{clear:both;} |
| - | # | + | #leftcol ul{padding-left:60px; padding-right:40px;} |
| - | # | + | #leftcol ul ul{padding-left:40px; padding-right:40px;} |
| - | # | + | #leftcol p{padding-left:0px; padding-right:40px;} |
| - | # | + | #leftcol .bigbutton p{padding-left:5px; padding-right:5px; padding-top:2px;} |
| - | .bigbutton{width: | + | .bigbutton{width:110px; height:50px; line-height:50px; font-size:1.2em; margin-right:10px; display:table;} |
.bigbutton a{display:block; height:100%;} | .bigbutton a{display:block; height:100%;} | ||
| Line 40: | Line 43: | ||
</html> | </html> | ||
| - | <div id=globalwrapper style="padding-left:20px; padding-right:20px"> | + | <div id=globalwrapper style="padding-left:20px; padding-right:20px; height:700px;"> |
<div id="leftcol" style="width:750px; float:left; overflow:auto;"> | <div id="leftcol" style="width:750px; float:left; overflow:auto;"> | ||
<html> | <html> | ||
| - | <h1> | + | <h1>Modelling - Optimal conditions</h1> |
| - | <div id="buttonrow" style="padding-top:30px; padding-bottom:70px; padding-left: | + | <div id="buttonrow" style="padding-top:30px; padding-bottom:70px; padding-left:10px; clear:both;"> |
<div class="bigbutton"> | <div class="bigbutton"> | ||
<a href="https://2013.igem.org/Team:Bielefeld-Germany/Modelling">Overview</a></div> | <a href="https://2013.igem.org/Team:Bielefeld-Germany/Modelling">Overview</a></div> | ||
| - | |||
| - | |||
<div class="bigbutton"> | <div class="bigbutton"> | ||
<a href="https://2013.igem.org/Team:Bielefeld-Germany/Modelling/Inter">Intermediates</a></div> | <a href="https://2013.igem.org/Team:Bielefeld-Germany/Modelling/Inter">Intermediates</a></div> | ||
| - | </div> | + | <div class="bigbutton"> |
| + | <p><a href="https://2013.igem.org/Team:Bielefeld-Germany/Modelling/Reduction">Mediator<br> Reduction</a></p></div> | ||
| + | <div class="bigbutton"> | ||
| + | <p><a href="https://2013.igem.org/Team:Bielefeld-Germany/Modelling/Oxidation">Mediator<br> Oxidation</a></p></div> | ||
| + | <div class="bigbutton"> | ||
| + | <p><a href="https://2013.igem.org/Team:Bielefeld-Germany/Modelling/Optimal">Optimal<br> conditions</a></p></div> | ||
| + | <div class="bigbutton"> | ||
| + | <p><a href="https://2013.igem.org/Team:Bielefeld-Germany/Modelling/Two_Reactions">Two<br> Reactions</a></p></div> | ||
</div> | </div> | ||
</html> | </html> | ||
| - | == | + | ==Overview== |
<p align="justify"> | <p align="justify"> | ||
| - | |||
The Microbial Fuel Cell will be subjected to numerous parameters, which will heavily influence its performance. Therefore, there is a need to inquiry the impact of those parameters on the efficiency of the system. In our approach we focused on how the reduction potential of the given mediators depends on two parameters, namely the pH value and the temperature. | The Microbial Fuel Cell will be subjected to numerous parameters, which will heavily influence its performance. Therefore, there is a need to inquiry the impact of those parameters on the efficiency of the system. In our approach we focused on how the reduction potential of the given mediators depends on two parameters, namely the pH value and the temperature. | ||
| - | |||
| - | |||
| - | |||
| + | All values were calculated based on the [http://en.wikipedia.org/wiki/Nernst_equation Nernst equation], which describes the relation between the electromotive force of the full cell and the temperature or the pH-value: | ||
</p> | </p> | ||
| - | |||
| - | |||
| - | |||
| - | == | + | [[File:Bielefeld-germany-model-optimal-Nernst.PNG|500px|center]]<br> |
| + | |||
| + | ==Optimal pH value== | ||
<p align="justify"> | <p align="justify"> | ||
| - | |||
The following exogenous mediators were analyzed under varying pH-value: | The following exogenous mediators were analyzed under varying pH-value: | ||
Neutral red, NADH, FADH2 and Methylene blue. The results are presented in the Figure 1. The optimal conditions for the growth of ''E.coli'' have been marked in red. Methylene blue has been chosen as the proof of concept for the experimental examination of the fuel cell, after various initial tests. Hence, it has been in the focus of the modeling of the performance as well. <!--The modeling, based on the equation (1) provides the following results--> | Neutral red, NADH, FADH2 and Methylene blue. The results are presented in the Figure 1. The optimal conditions for the growth of ''E.coli'' have been marked in red. Methylene blue has been chosen as the proof of concept for the experimental examination of the fuel cell, after various initial tests. Hence, it has been in the focus of the modeling of the performance as well. <!--The modeling, based on the equation (1) provides the following results--> | ||
| - | + | ||
The reduction potential for the optimal growth conditions for ''E.coli'', is between ~0.13 and ~0.8 V. The reduction potential for neutral red lies in this pH interval is between -0.4 and -0.7. This value is too low and would not lead to reduced performance of the fuel cell. The best electromotive force would be provided by the systems NADH and FADH2. | The reduction potential for the optimal growth conditions for ''E.coli'', is between ~0.13 and ~0.8 V. The reduction potential for neutral red lies in this pH interval is between -0.4 and -0.7. This value is too low and would not lead to reduced performance of the fuel cell. The best electromotive force would be provided by the systems NADH and FADH2. | ||
</p> | </p> | ||
| - | + | ||
[[Image:Bielefeld-germany-model-optimal-ph3.png.png|500px|thumb|center|'''Figure 1:''' Reduction potential of different mediators related to the pH value in the chamber. ]] | [[Image:Bielefeld-germany-model-optimal-ph3.png.png|500px|thumb|center|'''Figure 1:''' Reduction potential of different mediators related to the pH value in the chamber. ]] | ||
| - | |||
| - | == | + | |
| + | ==Optimal temperature== | ||
<p align="justify"> | <p align="justify"> | ||
| - | |||
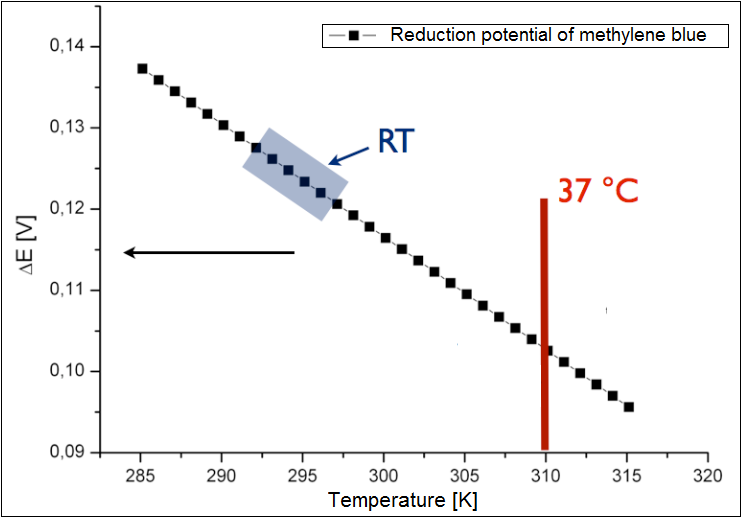
The analysis was conducted only for an exogenous mediator methylene blue. The results have been shown in the figure 2 below. It could be shown, that the calculated reduction potential differs only slightly for the broad spectrum of the temperature, and all lies at about 0.1 V. Therefor the temperature is not the limiting parameter for the performance of the fuel cell. The charge at the optimal growth condition for E. coli (37 °C or 310 K) is ~0.13 . | The analysis was conducted only for an exogenous mediator methylene blue. The results have been shown in the figure 2 below. It could be shown, that the calculated reduction potential differs only slightly for the broad spectrum of the temperature, and all lies at about 0.1 V. Therefor the temperature is not the limiting parameter for the performance of the fuel cell. The charge at the optimal growth condition for E. coli (37 °C or 310 K) is ~0.13 . | ||
</p> | </p> | ||
| - | + | ||
| + | |||
[[Image:Bielefeld-germany-model-optimal-temp.png|500px|thumb|center|'''Figure 2:''' Calculated reduction potential of the methylene blue dependent on the temperature of the system]] | [[Image:Bielefeld-germany-model-optimal-temp.png|500px|thumb|center|'''Figure 2:''' Calculated reduction potential of the methylene blue dependent on the temperature of the system]] | ||
| - | |||
| - | == | + | |
| + | |||
| + | ==Combined calculations for the proof system== | ||
<p align="justify"> | <p align="justify"> | ||
| - | |||
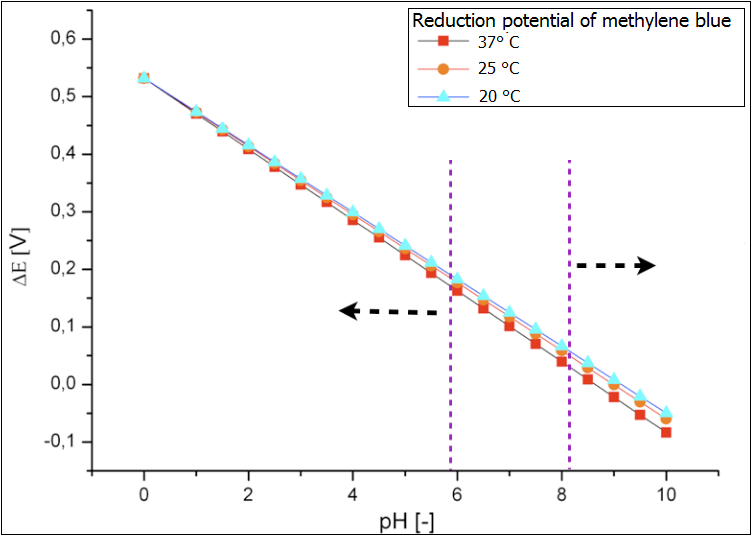
Ultimately the analysis of the influence of both temperature and pH value on the reduction potential of the proof system methylene blue has been performed. Its results are depicted in the figure 3. Again it could be shown that the temperature does not affect the reduction potential and therefore the effectiveness of the fuel cell. In contrast the pH-value in the chamber should be monitored and kept at the values that correspond to the optimal growth condition of the E.coli cells. The values below 6 would lead to too high reduction potential via the accumulation of the fermentation products under anaerob cultivation and decreased efficiency. Too basic conditions in the chamber (above 8) would lead to oxidation of the glucose and to so-called Blue-Bottle-Effect. The performance of the fuel cell would be also worse. | Ultimately the analysis of the influence of both temperature and pH value on the reduction potential of the proof system methylene blue has been performed. Its results are depicted in the figure 3. Again it could be shown that the temperature does not affect the reduction potential and therefore the effectiveness of the fuel cell. In contrast the pH-value in the chamber should be monitored and kept at the values that correspond to the optimal growth condition of the E.coli cells. The values below 6 would lead to too high reduction potential via the accumulation of the fermentation products under anaerob cultivation and decreased efficiency. Too basic conditions in the chamber (above 8) would lead to oxidation of the glucose and to so-called Blue-Bottle-Effect. The performance of the fuel cell would be also worse. | ||
| + | </p> | ||
| + | |||
| - | |||
[[Image:Bielefeld-germany-model-optimal-ph1.png|500px|thumb|center|'''Figure 3:''' Reduction potential of methylene blue dependent on pH value and temperature.]] | [[Image:Bielefeld-germany-model-optimal-ph1.png|500px|thumb|center|'''Figure 3:''' Reduction potential of methylene blue dependent on pH value and temperature.]] | ||
| - | <br> | + | |
| - | < | + | |
| + | |||
| + | |||
| + | <br><br><br><br> | ||
</div> | </div> | ||
| + | |||
| + | |||
| + | |||
<div id="asdf"> | <div id="asdf"> | ||
| Line 128: | Line 138: | ||
</div> | </div> | ||
| - | |||
</div> | </div> | ||
Latest revision as of 21:28, 27 October 2013
Modelling - Optimal conditions
Overview
The Microbial Fuel Cell will be subjected to numerous parameters, which will heavily influence its performance. Therefore, there is a need to inquiry the impact of those parameters on the efficiency of the system. In our approach we focused on how the reduction potential of the given mediators depends on two parameters, namely the pH value and the temperature. All values were calculated based on the [http://en.wikipedia.org/wiki/Nernst_equation Nernst equation], which describes the relation between the electromotive force of the full cell and the temperature or the pH-value:
Optimal pH value
The following exogenous mediators were analyzed under varying pH-value: Neutral red, NADH, FADH2 and Methylene blue. The results are presented in the Figure 1. The optimal conditions for the growth of E.coli have been marked in red. Methylene blue has been chosen as the proof of concept for the experimental examination of the fuel cell, after various initial tests. Hence, it has been in the focus of the modeling of the performance as well. The reduction potential for the optimal growth conditions for E.coli, is between ~0.13 and ~0.8 V. The reduction potential for neutral red lies in this pH interval is between -0.4 and -0.7. This value is too low and would not lead to reduced performance of the fuel cell. The best electromotive force would be provided by the systems NADH and FADH2.
Optimal temperature
The analysis was conducted only for an exogenous mediator methylene blue. The results have been shown in the figure 2 below. It could be shown, that the calculated reduction potential differs only slightly for the broad spectrum of the temperature, and all lies at about 0.1 V. Therefor the temperature is not the limiting parameter for the performance of the fuel cell. The charge at the optimal growth condition for E. coli (37 °C or 310 K) is ~0.13 .
Combined calculations for the proof system
Ultimately the analysis of the influence of both temperature and pH value on the reduction potential of the proof system methylene blue has been performed. Its results are depicted in the figure 3. Again it could be shown that the temperature does not affect the reduction potential and therefore the effectiveness of the fuel cell. In contrast the pH-value in the chamber should be monitored and kept at the values that correspond to the optimal growth condition of the E.coli cells. The values below 6 would lead to too high reduction potential via the accumulation of the fermentation products under anaerob cultivation and decreased efficiency. Too basic conditions in the chamber (above 8) would lead to oxidation of the glucose and to so-called Blue-Bottle-Effect. The performance of the fuel cell would be also worse.
 "
"