Template:Kyoto/Notebook/Sep 14
From 2013.igem.org
(Difference between revisions)
(→Restriction Enzyme Digestion) |
(→Ligation) |
||
| Line 113: | Line 113: | ||
!state||colspan="2"|Vector||colspan="2"|Inserter||Ligation High ver.2 | !state||colspan="2"|Vector||colspan="2"|Inserter||Ligation High ver.2 | ||
|- | |- | ||
| - | |experiment|| | + | |experiment||9/13 Pcon-PT181attenuater(SpeI&PstI)||1.7||9/13 RBS-lacZα-DT (XbaI & PstI)||12.5||5 |
|- | |- | ||
| - | |experiment|| | + | |experiment||9/13 pSB2C3 (XbaI & PstI)||1.9||9/14 RpaB (XbaI & PstI)||16.3||20.1 |
|- | |- | ||
| - | |experiment|| | + | |experiment||9/13 pSB2C3 (XbaI & PstI)||4.7||9/14 PkaiBC (EcoRI & SpeI)||6.7||11.4 |
|- | |- | ||
| - | |experiment|| | + | |experiment||9/14 RBS-GFP-DT (PstI&XbaI)||0.7||9/14 PkaiBC (EcoRI & SpeI)||2.6||3.3 |
|- | |- | ||
| - | |experiment|| | + | |experiment||9/14 Plac (SpeI&PstI)||2.4||9/14 RBS-lysis1-DT (XbaI & PstI)||4.9||7.3 |
|- | |- | ||
| - | |experiment|| | + | |experiment||9/14 Plac (SpeI&PstI)||2.4||9/14 RBS-lysis2-DT (XbaI & PstI)||7.0||9.4 |
|- | |- | ||
| - | |experiment|| | + | |experiment||9/14 Plac (SpeI&PstI)||2.4||9/14 RBS-lysis3-DT (XbaI & PstI)||9.2||11.6 |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
|} | |} | ||
Samples were evaporeted used evaporator into about 7 µL | Samples were evaporeted used evaporator into about 7 µL | ||
</div> | </div> | ||
| - | |||
===Colony PCR=== | ===Colony PCR=== | ||
Revision as of 13:56, 25 September 2013
Contents |
Sep 14
Restriction Enzyme Digestion
| 9/14 pSB4K5S | EcoRI | XbaI | SpeI | PstI | BufferB | BufferD | BSA | MilliQ | total | |
|---|---|---|---|---|---|---|---|---|---|---|
| 2cuts | 10µL | 1µL | 0µL | 1µL | 0µL | 3µL | 0µL | 3µL | 12µL | 30µL |
| NC | 0.5µL | 0µL | 0µL | 0µL | 0µL | 1µL | 0µL | 1µL | 7.5µL | 10µL |
| 9/13 Plac | XbaI | PstI | BufferH | BSA | MilliQ | total | |
|---|---|---|---|---|---|---|---|
| 1 cut | 9µL | 0µL | 1µL | 3µL | 3µL | 14µL | 30µL |
| NC | 1µL | 0µL | 0µL | 1µL | 1µL | 7µL | 10µL |
| 9/14 PKaiBC | EcoRI | XbaI | SpeI | PstI | BufferB | BSA | MilliQ | total | |
|---|---|---|---|---|---|---|---|---|---|
| 2cuts | 11µL | 1µL | 0µL | 1µL | 0µL | 3µL | 3µL | 11µL | 30µL |
| NC | 4µL | 0µL | 0µL | 0µL | 0µL | 1µL | 1µL | 4µL | 10µL |
| 9/14 RpaB | EcoRI | XbaI | SpeI | PstI | BufferD | BSA | MilliQ | total | |
|---|---|---|---|---|---|---|---|---|---|
| 2cuts | 13µL | 0µL | 1µL | 0µL | 1µL | 3µL | 3µL | 9µL | 30µL |
| NC | 2µL | 0µL | 0µL | 0µL | 0µL | 1µL | 1µL | 5µL | 10µL |
| 8/17 RBS-GFP-DT | EcoRI | XbaI | SpeI | PstI | BufferD | BSA | MilliQ | total | |
|---|---|---|---|---|---|---|---|---|---|
| 1cut | 9µL | 0µL | 1µL | 0µL | 0µL | 3µL | 3µL | 14µL | 30µL |
| NC | 0.5µL | 0µL | 0µL | 0µL | 0µL | 1µL | 1µL | 7.5µL | 10µL |
- incubated 37°C 1hour
Liquid Culture
| Sample | medium |
|---|---|
| 9/11 Plac(BBa-R0011)-(3) | Plusgrow medium(+Amp) |
PCR
| genome DNA | MilliQ | Big Dye | 5x buffer | temp(400mg) | primer | total | |
|---|---|---|---|---|---|---|---|
| Pcon-RBS-luxR-DT | 0.5 | 1.75 | 1.75 | 0.4 | 0.5 | 5.1 | 10.5 |
| Pcon-RBS-luxR-DT | 0.5 | 1.75 | 1.75 | 0.4 | 0.5 | 5.1 | 10.5 |
| Pcon-RBS-tetR-DT | 0.5 | 1.75 | 1.75 | 3 | 0.5 | 3 | 10.5 |
| Pcon-RBS-tetR-DT | 0.5 | 1.75 | 1.75 | 3 | 0.5 | 3 | 10.5 |
| Pcon-RBS-luxR-DT | 0.5 | 1.75 | 1.75 | 2.4 | 0.5 | 3.6 | 10.5 |
| Plux-RBS-GFP-DT | 0.5 | 1.75 | 1.75 | 1.9 | 0.5 | 4.1 | 10.5 |
| Plux-RBS-GFP-DT | 0.5 | 1.75 | 0.5 | 1.9 | 0.5 | 4.1 | 10.5 |
| Pcon-RBS-GFP-DT | 0.5 | 1.75 | 0.5 | 1.2 | 0.5 | 4.8 | 10.5 |
| RBS-lysis3-DT | 0.5 | 1.75 | 0.5 | 1.0 | 0.5 | 5.0 | 10.5 |
| Pcon-RBS-GFP-DT | 0.5 | 1.75 | 0.5 | 1.4 | 0.5 | 4.6 | 10.5 |
| Pcon-RBS-GFP-DT | 0.5 | 1.75 | 0.5 | 1.4 | 0.5 | 4.6 | 10.5 |
| RBS-lysis3-DT | 0.5 | 1.75 | 1.75 | 0.4 | 0.5 | 5.1 | 10.5 |
| Ptet-RBS-spinach-DT | 0.5 | 1.75 | 0.5 | 0.59 | 0.5 | 0.1 | 10.5 |
| PreDenature | Denature | Annealing | Extension | cycle |
|---|---|---|---|---|
| 96°C | 96°C | 50°C | 60°C | -- |
| 2min | 10s | 5s | 4min | 30cycle |
Restriction Enzyme Digestion
| 9/14 Plac(1A2) | EcoRI | XbaI | SpeI | PstI | BufferB | BSA | MilliQ | total | |
|---|---|---|---|---|---|---|---|---|---|
| 2cuts | 23µL | 0µL | 0µL | 0.3µL | 0µL | 3µL | 3µL | 0µL | 30µL |
| 9/14 RBS-GFP-DT | EcoRI | XbaI | SpeI | PstI | BufferH | BSA | MilliQ | total | |
|---|---|---|---|---|---|---|---|---|---|
| 2 cuts | 23µL | 1µL | 0µL | 0µL | 0µL | 3µL | 3µL | 0µL | 30µL |
Ligation
| state | Vector | Inserter | Ligation High ver.2 | ||
|---|---|---|---|---|---|
| experiment | 9/13 Pcon-PT181attenuater(SpeI&PstI) | 1.7 | 9/13 RBS-lacZα-DT (XbaI & PstI) | 12.5 | 5 |
| experiment | 9/13 pSB2C3 (XbaI & PstI) | 1.9 | 9/14 RpaB (XbaI & PstI) | 16.3 | 20.1 |
| experiment | 9/13 pSB2C3 (XbaI & PstI) | 4.7 | 9/14 PkaiBC (EcoRI & SpeI) | 6.7 | 11.4 |
| experiment | 9/14 RBS-GFP-DT (PstI&XbaI) | 0.7 | 9/14 PkaiBC (EcoRI & SpeI) | 2.6 | 3.3 |
| experiment | 9/14 Plac (SpeI&PstI) | 2.4 | 9/14 RBS-lysis1-DT (XbaI & PstI) | 4.9 | 7.3 |
| experiment | 9/14 Plac (SpeI&PstI) | 2.4 | 9/14 RBS-lysis2-DT (XbaI & PstI) | 7.0 | 9.4 |
| experiment | 9/14 Plac (SpeI&PstI) | 2.4 | 9/14 RBS-lysis3-DT (XbaI & PstI) | 9.2 | 11.6 |
Samples were evaporeted used evaporator into about 7 µL
Colony PCR
| Sample | base pair |
|---|---|
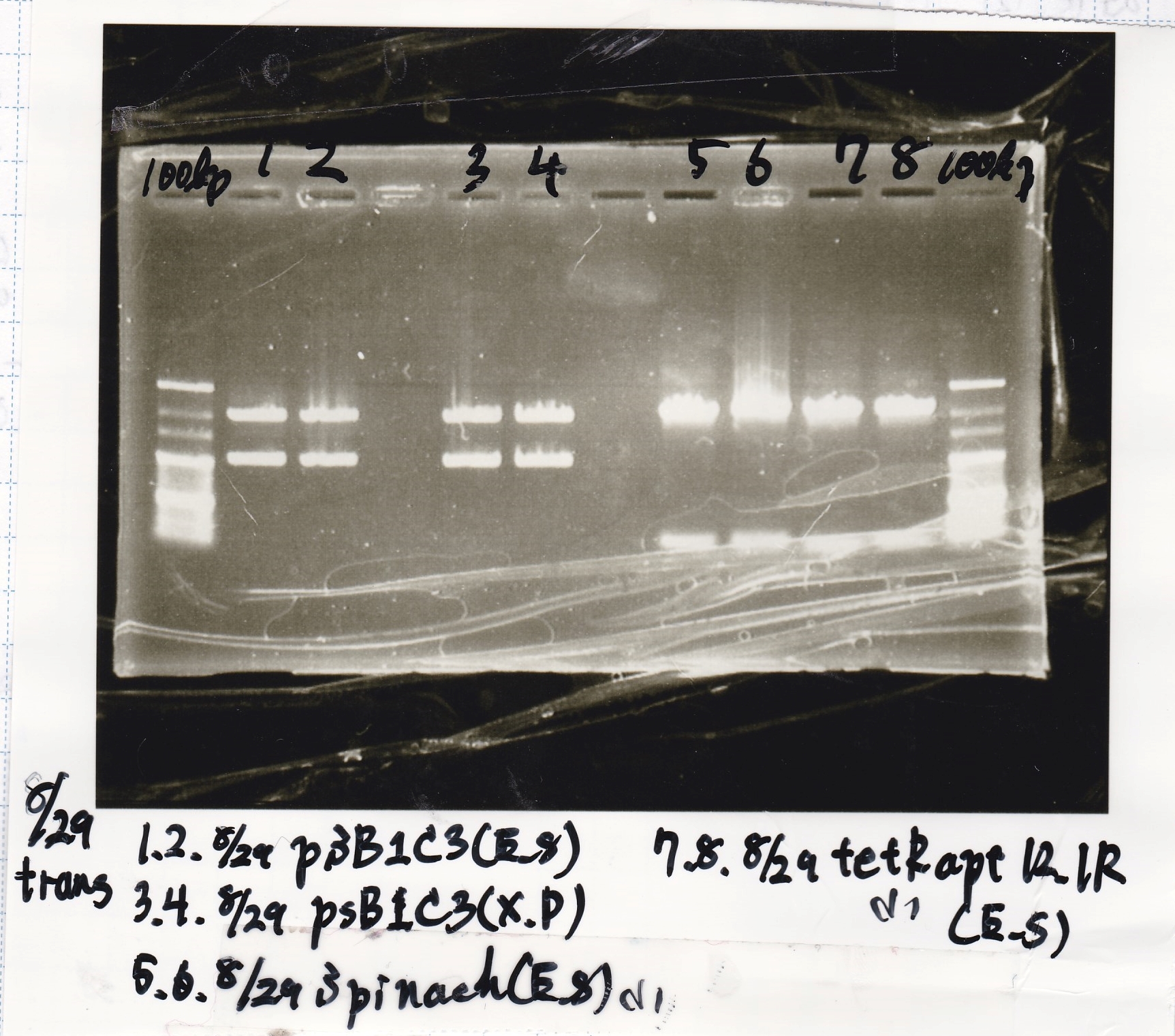
| 8/29 Pcon-RBS-GFP-DT-Pcon-RBS-luxR-DT(8/20 LB Amp) (1)~(4) | 2143 |
| 8/29 Plux-RBS-GFP-DT(8/27 LB-CP) (1)~(2) | 1227 |
| 8/29 Pbad/araC-RBS-RFP{BBa_I13516} (8/21 LB-CP) (1)~(2) | 2256 |
| 8/29 RBS-lysis3-DT-(1) | 1210 |
| PreDenature | Denature | Annealing | Extension | cycle |
|---|---|---|---|---|
| 94°C | 94°C | 55°C | 68°C | -- |
| 5min | 30s | 30s | 2min | 30cycle |
Liquid Culture
| Sample | medium |
|---|---|
| 8/29 Pcon-RBS-GFP-DT-Pcon-RBS-luxR-DT-(1) | Plusgrow medium(+Amp) |
| 8/29 Pcon-RBS-GFP-DT-Pcon-RBS-luxR-DT-(2) | Plusgrow medium(+Amp) |
| 8/29 Pcon-RBS-GFP-DT-Pcon-RBS-luxR-DT-(3) | Plusgrow medium(+Amp) |
| 8/29 Pcon-RBS-GFP-DT-Pcon-RBS-luxR-DT-(4) | Plusgrow medium(+Amp) |
| 8/29 Plux-RBS-GFP-DT-(1) | Plusgrow medium(+CP) |
| 8/29 Plux-RBS-GFP-DT-(2) | Plusgrow medium(+CP) |
| 8/29 Pbad/araC-RBS-RFP-DT-(1) | Plusgrow medium(+CP) |
| 8/29 Pbad-araC-RBS-RFP-DT-(2) | Plusgrow medium(+CP) |
| RBS-lysis3-DT-(1) | Plusgrow medium(+CP) |
Transformation
| Name | Sample | Competent Cells | Total | Plate |
|---|---|---|---|---|
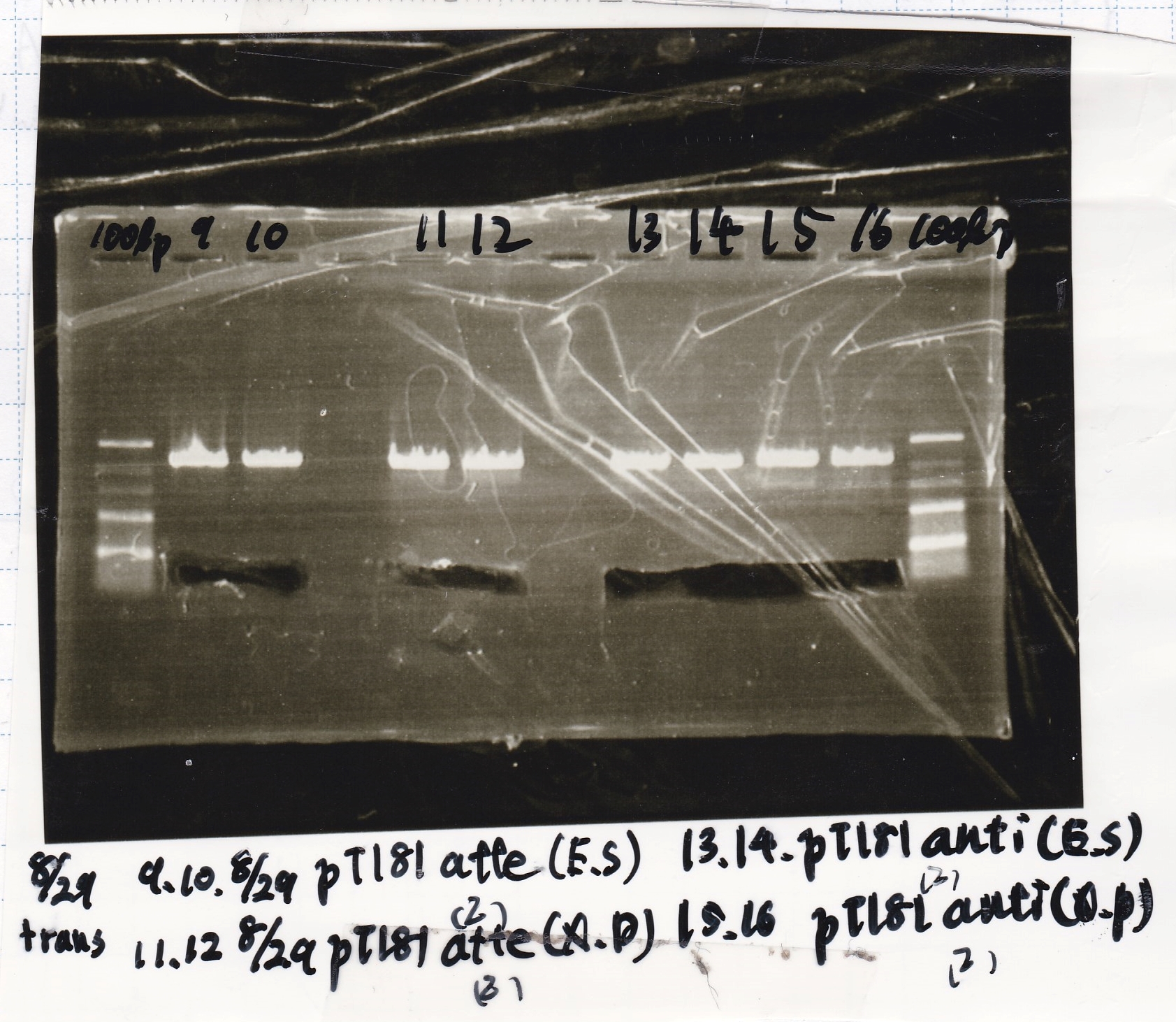
| 8/27 pT181 attenuator-DT | 2µL | 20µL | 22µL | CP |
| 8/27 RBS-lysis3-DT | 2µL | 20µL | 22µL | CP |
| 8/28 RBS-lysis3-DT | 2µL | 20µL | 22µL | CP |
| 8/28 Pcon-RBS-GFP-DT-Pcon-RBS-LuxR-DT | 2µL | 20µL | 22µL | Amp |
| 8/28 RBS-lysis1-DT | 2µL | 20µL | 22µL | CP |
| 8/28 Plux-RBS-GFP-DT | 2µL | 20µL | 22µL | CP |
| 8/28 Spinach-DT | 2µL | 20µL | 22µL | CP |
PCR
| genome DNA | KOD plus | 10x buffer | dNTP | MgSO4 | SasA_fwd primer | SasA_rev primer | MilliQ | total |
|---|---|---|---|---|---|---|---|---|
| 0.5 | 2.5 | 2.5 | 1.5 | 0.75 | 0.75 | 25 |
| PreDenature | Denature | Annealing | Extension | cycle |
|---|---|---|---|---|
| °C | °C | °C | °C | -- |
| (◎Д◎) |
 "
"