Team:Bielefeld-Germany/Modelling/Optimal
From 2013.igem.org
m |
m |
||
| Line 79: | Line 79: | ||
=='''Optimal pH value '''== | =='''Optimal pH value '''== | ||
<p align="justify"> | <p align="justify"> | ||
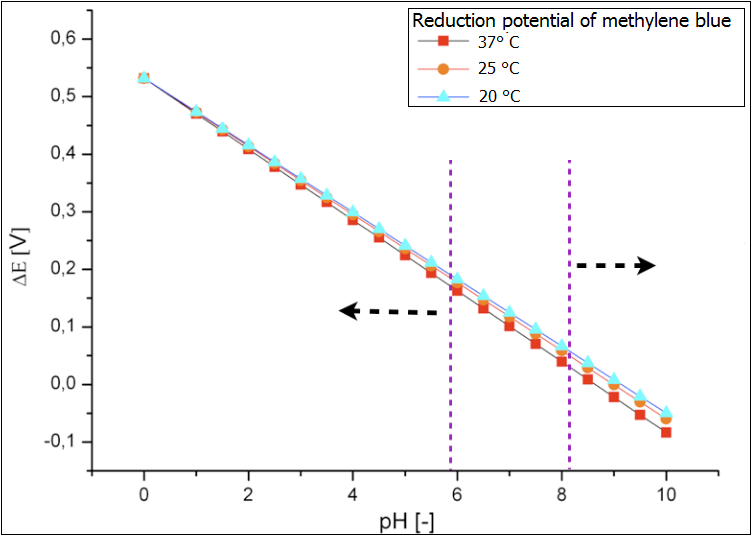
| - | + | Following exogenous mediators were analysed under the variating pH-value: | |
| - | + | Neutral red NADH FADH2 and Methylene blue. The results are presented in the Figure 1. The optimal conditions for the growth of E.coli have been marked in light-red. Methylene blue has been chosen as the proof system for the experimental examination of the fuel cell. Hence, it has been in the focus of the modeling of the performance as well. Its reduction potential, for the optimal growth conditions for E.coli, is between ~0.13 and ~0.8 V. The reduction potential for the neutral red lies in this pH-value interval between -0.4 and -0.7. This value could be too low and wouldn’t lead to reduced performance of the fuel cell. The best electromotive force would be provided by the systems NADH and FADH2. | |
</p> | </p> | ||
| + | |||
<br> | <br> | ||
| - | [[Image:Bielefeld-germany-model-optimal- | + | [[Image:Bielefeld-germany-model-optimal-ph3.png.png|500px|thumb|center|'''Figure 1:''' Reduction potential of different mediators related to the pH value in the chamber. ]] |
<br><br><br><br><br> | <br><br><br><br><br> | ||
| - | [[Image:Bielefeld-germany-model-optimal- | + | [[Image:Bielefeld-germany-model-optimal-ph1.png|500px|thumb|center|'''Figure 2:''' title ]] |
| + | |||
| + | |||
<br> | <br> | ||
Revision as of 23:10, 4 October 2013
Modeling
Overview
The microbial fuel cell will be subjected to numerous parameters, which will heavily influence its performance. Therefore, there is a need to inquiry the impact of those parameters on the efficiency of the system. In our approach we focused on how the reduction potential of the given mediators depends on two most significant parameters, namely the pH value and the temperature. All values were calculated based on the [http://en.wikipedia.org/wiki/Nernst_equation Nernst equation], which describes the relation between the electromotive force of the full cell and the temperature or the pH-value.
Optimal pH value
Following exogenous mediators were analysed under the variating pH-value: Neutral red NADH FADH2 and Methylene blue. The results are presented in the Figure 1. The optimal conditions for the growth of E.coli have been marked in light-red. Methylene blue has been chosen as the proof system for the experimental examination of the fuel cell. Hence, it has been in the focus of the modeling of the performance as well. Its reduction potential, for the optimal growth conditions for E.coli, is between ~0.13 and ~0.8 V. The reduction potential for the neutral red lies in this pH-value interval between -0.4 and -0.7. This value could be too low and wouldn’t lead to reduced performance of the fuel cell. The best electromotive force would be provided by the systems NADH and FADH2.
Optimal temperature
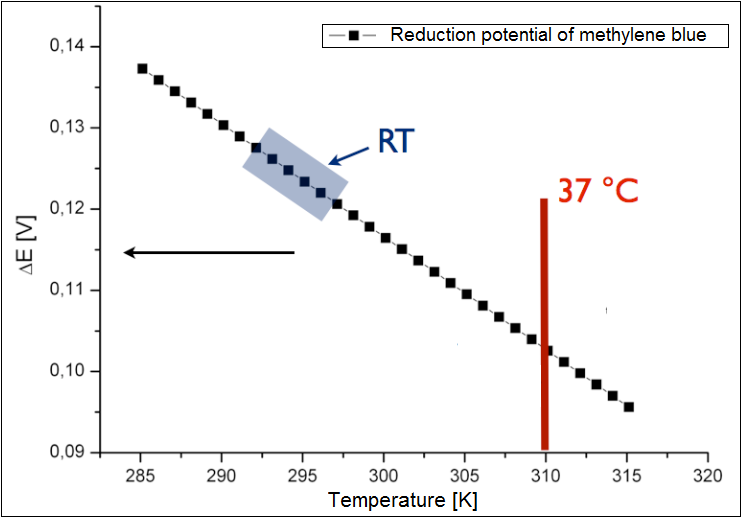
The analysis was conducted only for an exogenous mediator methylene blue. The results have been shown in the figure 3 below. It could be shown, that the calculated reduction potential differs only slightly for the broad spectrum of the temperature, and all lies at about 0.1 V. Therefor the temperature is not the limiting parameter for the performance of the fuel cell. The charge at the optimal growth condition for E. coli (37 °C or 310 K) is ~0.13 .
References
 "
"