Team:Bielefeld-Germany/Project/Nanowires
From 2013.igem.org
Nanowires
Overview
Multiple bacteria form special, electrically highly conductive pili, which are required for survival in anaerobic environments. Electrons, generated through the oxidation of different substrates can be transported by these pili and transferred to alternative solid electron-acceptors, such as sulfur or iron compounds. These properties characterize them as an interesting option for the optimization of E.coli for the use in MFCs. These so called nanowires are of special interest because they could increase the number of bacteria contacting the surface of the anode. Unfortunately multiple genes, arranged in large gene clusters are required to form nanowires in organisms such as Geobacter sulfurreducens, so that the cloning of a functional expression system seems to be a big challenge. Therefore the genetic modification of the existing non-electrically conductive type 4 pili from E. coli with the objective to provide them with electrically conductive properties is a very interesting option to utilize E. coli as a MFC-organism.
Theory
Besides the well known group of F-pili responsible for conjugal gene transfer, several other types of these hair-like structures can be found. They fulfill a variety of different functions. The pilus classification is based on specific host-receptor interactions, verifiability with different antibodies and the amino acid sequence of the pilin protein, a conserved oligomeric element, found in every pili-structure (Strom and Lory., 1997). One of these families, containing the so called Type IV pilis, seems suitable for building a microbial fuel cell, because some of these protein structures are able to conduct electrons. In some anaerobic habitats bacteria depend on the transfer of electrons to electron acceptors like Fe(III)oxides to dump the electrons generated during their metabolic activities. The insolubility of electron acceptors like Fe2O3 led to the development of a complex network of conductive structures, which electrically connect the bacteria with their environment (Shi et al., 2007). Based on these properties the use of biocompatible electrodes could enable the bacteria to perform anaerobic respiration with a simultaneous use of the released electrons for the production of electricity.
In microbial fuel cells filled with waste water and inoculated with sewage sludge a formation of a thick biofilm is observed at the anode under anaerobic conditions. The biofilm contains a mixed culture of characteristic organisms. Besides species like Shewanella putrefaciens and Rhodoferax ferrireducens mainly representatives of the genus Geobacter are found, which are capable of using an electrode as their final electron acceptor (Kim et al., 2005). In particular Geobacter sulfurreducens is well known for its ability to reduce Fe(III)Oxides and other insoluble compounds by the formation of cytochromes in the outer membrane and the production of electrically conductive pili, which are also called nanowires. Many studies have been conducted to evaluate the special properties of this group of anaerobe bacteria resulting in Geobacter sulfurreducens to become the model organism due to its genetic accessibility and because it is one of the first fully sequenced organisms of this group. Therefore use of the pili formation gene complex from this species to modify the target organism Escherichia coli with regard to the production of electrically conductive pili (Coppi et al., 2001) appeared to be the best approach.
In contrast to the use of mediators, which cause an indirect electron transfer, the use of nanowires could enable Escherichia coli to use direct anaerobic respiration without limiting effects like diffusion or the electron transfer between redox-mediator and anode. Compared to cytochromes which also would allow for anaerobic respiration by direct cell-anode contact, nanowires allow much more bacteria to connect to an electrode.
Although a promising target from a purely mechanistical point of view, the proposed use of genetically engineered Escherichia coli Type IV pili by changing the amino acid sequence with regard to an increased content of aromatic amino acids was not attempted because of major biosafety concerns: all existing type IV pili from Escherichia coli (Vargas et al., 2013) are associated with pathogenicity. As a microbial fuel cell system should be free from any safety problems, this pili type is not suitable for our purposes. In contrast, the type IV pili of G. sulfurreducens are not associated with pathogenicity, thus making the pili-coding genes from this risk group 1 organism a viable alternative for the nanowire project.
Genetic Approach
In general all pili-like structures consist mainly of oligomeric pilin-proteins. For Geobacter sulfurreducens the corresponding gene is called pilA and contains 273 nucleotides. Previous studies pointed out that next to this pivotal gene at least one big cluster of other genes is essential for the functional expression of the Geobacter-nanowires. This was shown by complementing a pilA knockout through a plasmid carrying pilA gene. Without the entire operon GSU 1496-1505 it was not possible to complement the knockout regarding a functional nanowire expression. Furthermore the genes pilB, pilT, pilC, pilS and pilR carry out important tasks concerning a functional PilA expression, like the regulation of pilA transcription (Richter et al., 2012). The relevant loci are illustrated in figure 2.
Because of its size of 16362 bp, the gene cluster GSU 1491-1505 was divided into two parts of 7053 and 8835 bp to permit the use of the Gibson Assembly. This is essential to bring the cluster into the BioBrick form because the region contains multiple forbidden restriction sites for EcoRI and PstI prohibiting use of the standard BioBrick Assembly. Further studies revealed that PilA translation in G. sulfurreducens occurs from two functional translation start codons for pilA, resulting in two isoforms of the PilA protein. Both were shown to be essential for the formation of functional nanowires (Richter et al., 2012). Therefore, two different versions of the GSU 1496-1505 operon were tested to analyze its functionality in Escherichia coli, differing in the existence of the natural G. sulfurreducencs promoter.
Because similarity based searches with all fifteen genes revealed no similar genes within the Escherichia coli genome, the insertion of the whole region containing GSU 1491-1505 in the high copy plasmid pSB1C3 is necessary and should enable E. coli to produce functional nanowires.
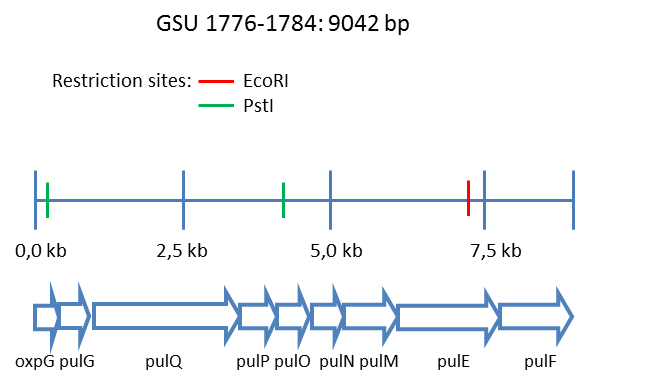
Since there were no information available whether the pilin secretion system of the type IV pili producing E. coli strains exists in the strain E. coli KRX another Geobacter sulfurreducens operon, containing the respective secretion proteins, might be relevant for a functional expression (Reguera et al. 2005). The gene structure of this cluster of 9042 bp is shown in Figure 3.
Results
After the successful amplification of the Geobacter sulfurreducens clusters GSU 1491-1495, GSU 1496-1505 and GSU Promoter 1496-1505, assembly of the DNA sequences into a BioBrick failed several times. Due to the large number of illegal restriction sites and the not very promising prospects for a functional expression of nanowires in Escherichia coli, due to the large number of essential proteins and the complex promoter-structure, the nanowire project was stopped to focus on the other projects.
References
- Coppi, M. V., Leang, C., Sandler, S. J., & Lovley, D. R. (2001). Development of a Genetic System for Geobacter sulfurreducens. [http://aem.asm.org/content/67/7/3180.short Applied and environmental microbiology, 67](7), 3180-3187.
- Kim, J. R., Min, B., & Logan, B. E. (2005). Evaluation of procedures to acclimate a microbial fuel cell for electricity production. [http://link.springer.com/article/10.1007/s00253-004-1845-6 Applied microbiology and biotechnology, 68](1), 23-30.valuation of procedures to acclimate a microbial fuel cell for electricity production.
- Reguera, G., McCarthy, K. D., Mehta, T., Nicoll, J. S., Tuominen, M. T., & Lovley, D. R. (2005). Extracellular electron transfer via microbial nanowires. [http://www.nature.com/nature/journal/v435/n7045/abs/nature03661.html Nature, 435](7045), 1098-1101.
- Richter, L. V., Sandler, S. J., & Weis, R. M. (2012). Two isoforms of Geobacter sulfurreducens PilA have distinct roles in pilus biogenesis, cytochrome localization, extracellular electron transfer, and biofilm formation. [http://jb.asm.org/content/194/10/2551.short Journal of bacteriology, 194](10), 2551-2563.
- Shi, L., Squier, T. C., Zachara, J. M., & Fredrickson, J. K. (2007). Respiration of metal (hydr) oxides by Shewanella and Geobacter: a key role for multihaem c‐type cytochromes. [http://onlinelibrary.wiley.com/doi/10.1111/j.1365-2958.2007.05783.x/full Molecular microbiology, 65](1), 12-20.
- Strom, M. S., & Lory, S. (1993). Structure-function and biogenesis of the type IV pili. [http://www.annualreviews.org/doi/pdf/10.1146/annurev.mi.47.100193.003025 Annual Reviews in Microbiology, 47](1), 565-596.
- Vargas, M., Malvankar, N. S., Tremblay, P. L., Leang, C., Smith, J. A., Patel, P., ... & Lovley, D. R. (2013). Aromatic amino acids required for pili conductivity and long-range extracellular electron transport in Geobacter sulfurreducens. [http://mbio.asm.org/content/4/2/e00105-13.short mBio, 4](2).
 "
"