Team:Heidelberg/Templates/Delftibactin week22
From 2013.igem.org
Contents |
23-09 - 29-09-13
Evaluation of Background Expression and Indcibility of Possible Target Strains
Electroporation of mRFP Controls
1 µl miniprpep DNA of the backbones pSB6A1 and pSB3C5 containing mRFP were elctroporated in E.coli Bl21 DE3. Cells were streaked on LB Amp + Chlor plates and stored at RT for two days.
Result
All plates were heavily overgrown. As discussed next, the obtained BL21 DE3 were actually BL21 DE3 pLys and therefore resitant to chloramphenicol. Electroporation has to be repeated, after real E. coli BL21 DE3 are obtained.
New BL21 DE3
We obtained new BL21 DE3 from the Russel Lab, which were obtained from NEB directly. Cells were inocculated in 10 ml LB and streaked on LB Chlor plates. The vial was frozen again at -80°C.
Electroporation of mRFP Controls
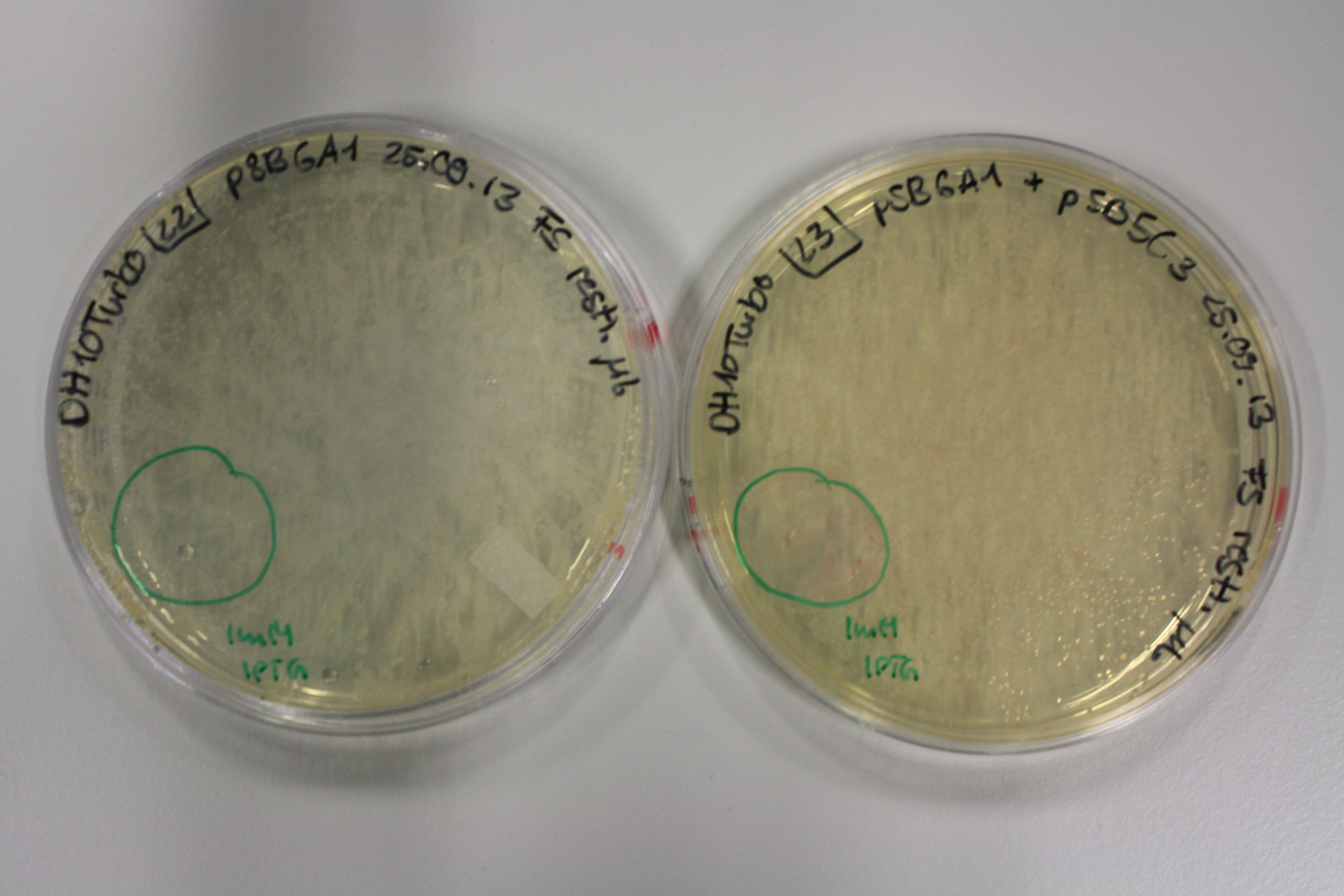
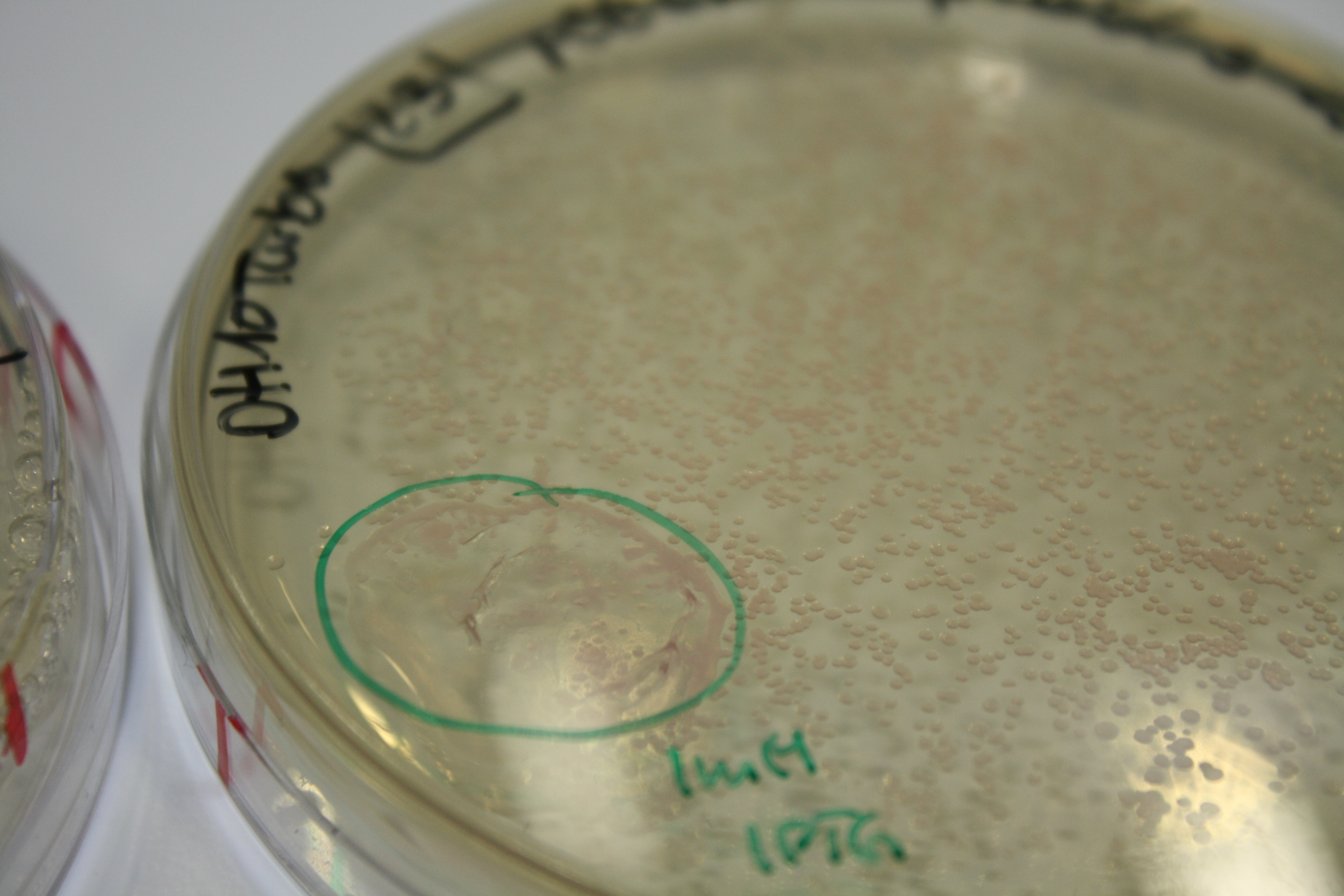
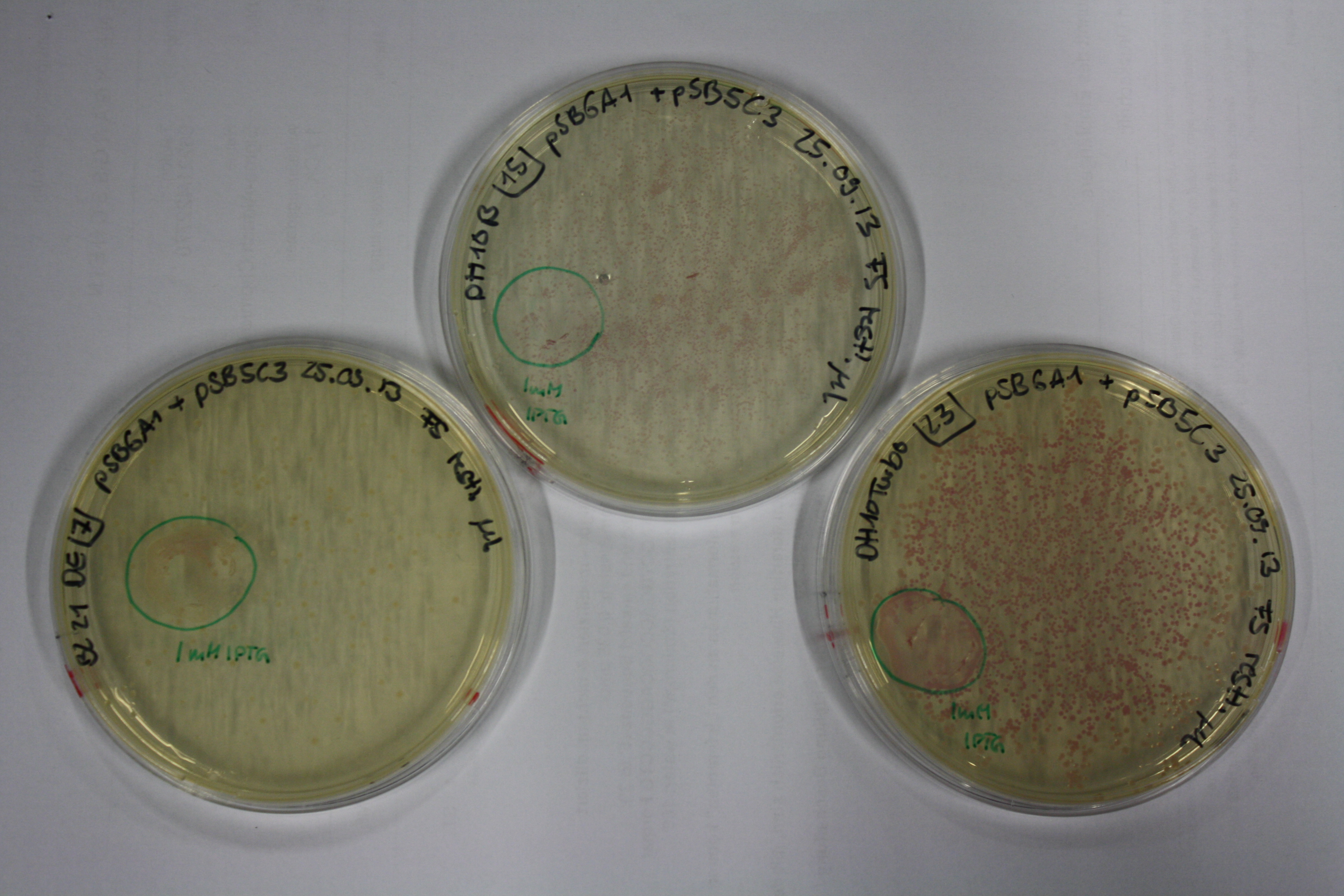
Real E. coli BL21 DE3, DH10ß and NEB Turbo were co-electroporated with 1 µl miniprpep DNA of the backbones pSB6A1 and pSB3C5 containing mRFP to check for leaky ecpression. Transformed bacteria were streaked on 2YT plates with Amp + Chlor and incubated ON at 30°C.
The next day, expression of mRFP on backbones pSB6A1 and pSB3C5 was induced by 50 µl 1 mM IPTG as well as inocculated in YT Amp + Chlor + 1 mM IPTG.
Result
After 6 h, only BL21 DE showed expression of mRFP. Yet, it was not restricted to the induced area. Another 10 h later, all strains showed red colonies with and without induction. E. coli BL21 DE3 showed least background expression and intermediate expression upon induction on plate and in liquid media.
Electrocompetent E. coli BL21 DE + DelRest and pIK8.6
Electroporation of DelRest, DelH and pIK8.6
As stated above, BL21 DE3 showed least background expression of mRFP and were therefore chosen for preparation of electrocompetent cells as described. Cells were streaked on 2YT Kan + Chlor plates and incubated at RT for 2 days.
Colony-PCR Conditions CP.W22.A
6 colonies were analyzed for Del Rest and pIK8.6 by colony PCR.
- DelL
| Reagent | Amount [µl] |
|---|---|
| Colonies | - |
| FS_14 10 µM | 1 |
| FS_15 10 µM | 1 |
| OneTaq Master Mix | 10 |
| DMSO | 1 |
| ddH2O | 7 |
| Cycles | Temperature [°C] | Time [s] |
|---|---|---|
| 1 | 95 | 300 |
| 12 | 95 | 30 |
| 66 ↓ 0.5 | 30 | |
| 72 | 90 | |
| 18 | 95 | 30 |
| 63 | 30 | |
| 72 | 90 | |
| 1 | 72 | 720 |
| 1 | 12 | inf |
- pIK8.6
| Reagent | Amount [µl] |
|---|---|
| Colonies | - |
| VF2 10 µM | 1 |
| IK_25 10 µM | 1 |
| OneTaq Master Mix | 10 |
| DMSO | 1 |
| ddH2O | 7 |
| Cycles | Temperature [°C] | Time [s] |
|---|---|---|
| 1 | 95 | 300 |
| 35 | 95 | 30 |
| 54 | 30 | |
| 72 | 90 | |
| 1 | 72 | 600 |
| 1 | 12 | inf |
Result
Expected band: ~1.4 Kb (DelL) and ~1 Kb (pIK8.6)
All of the analyzed colonies shows expected band for DelL.
- => Inocculate 2 ml 2YT Kan + Chlor with 2 colonies for preparation of electrocompetent cells
Electrocompetent Cells
Electrocompetent E. coli BL21 DE3 + DelRest + pIK8.6 wer prepared as described.
 "
"