From 2013.igem.org
Amplifications
Amplification of fragment 1
A
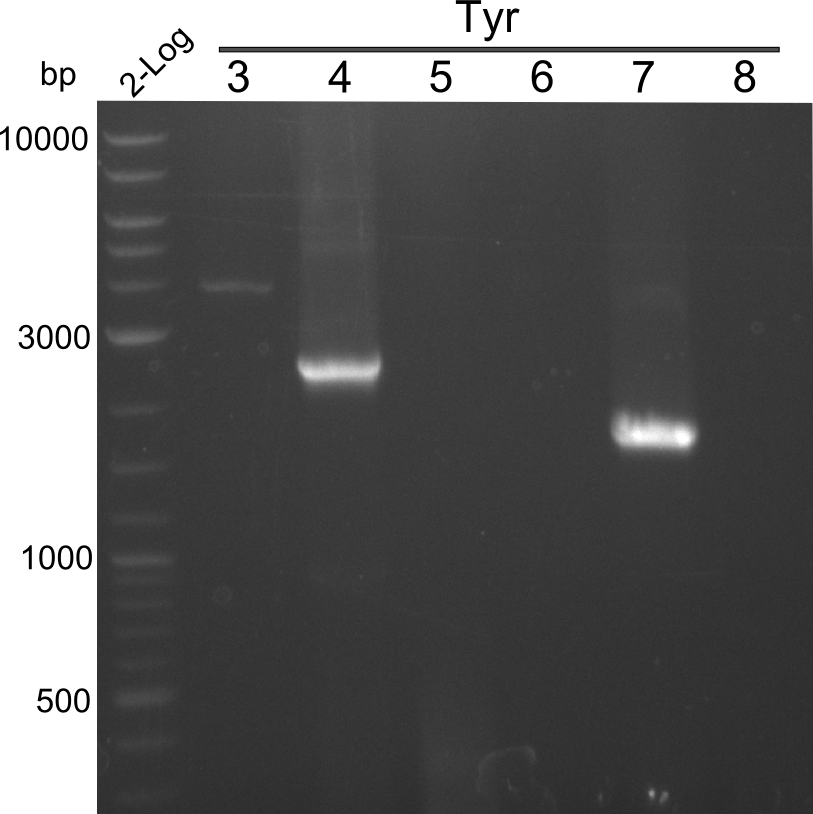
PCR Results of 25.07.13 2-log ladder and Tyr 3-8 with Q5
| what | µl
|
| pSB1C3 | 0,5
|
| IK22 | 2
|
| IK23 | 2
|
| Q5 2x Master mix | 10
|
| ddH20 | 5,5
|
| Cycles | temperature [°C] | Time [s]
|
| 1 | 98 | 120
|
| 35 | 98 | 5
|
| 66 | 10
|
| 72 | 120
|
| 1 | 72 | 600
|
| 1 | 12 | inf
|
Result
band in gel--> cut and extraction
B
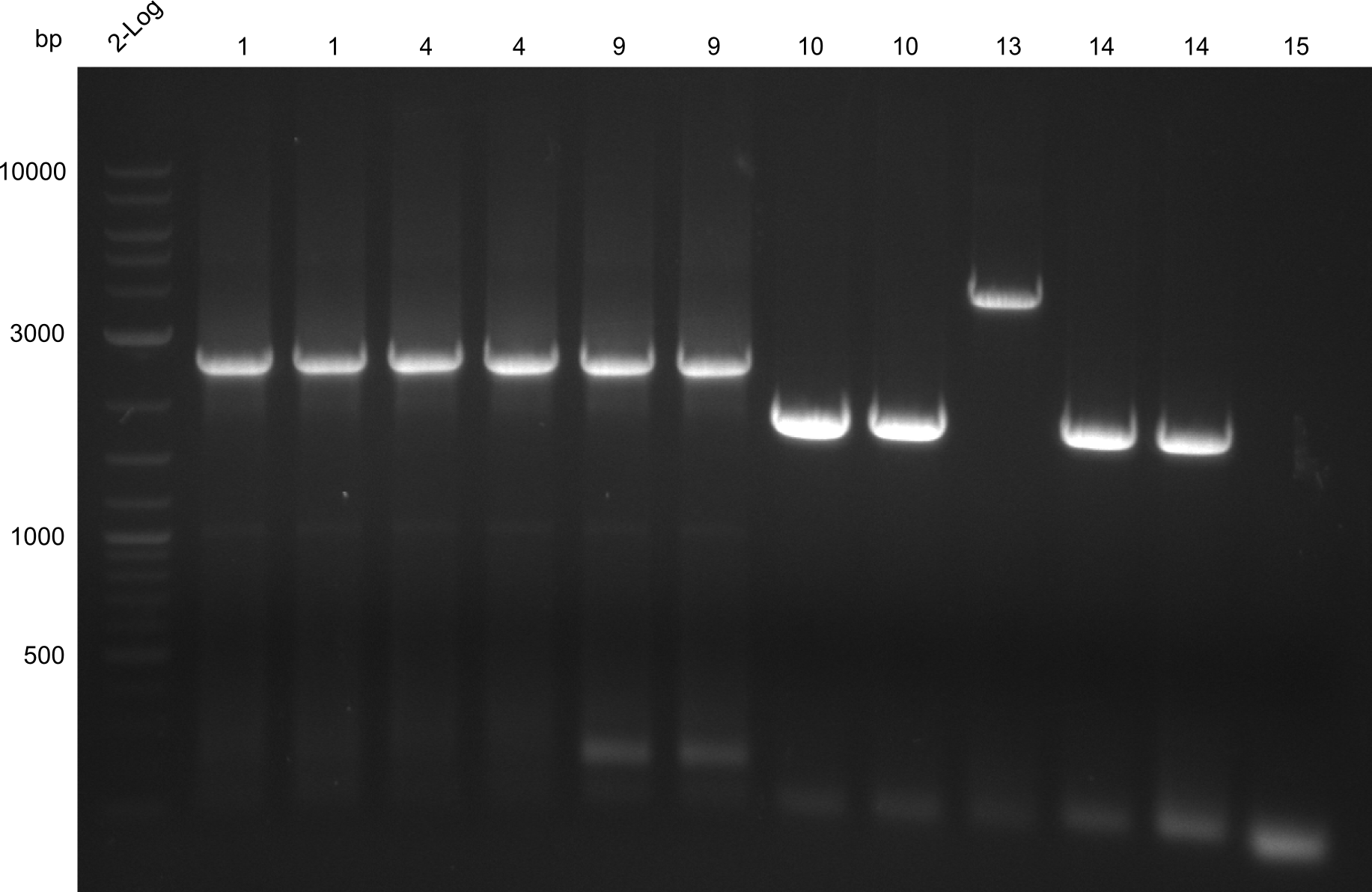
all constructs extracted by gel extraction; fragment 15 missing -> Optimization
| what | µl
|
| IK23 | 2
|
| IK22 | 2
|
| pSB1C3 | 0.5 (for fragments 1, 4 & 9)
|
| Q5 2x Master mix | 10
|
| ddH20 | 5
|
| Cycles | temperature [°C] | Time [min:s]
|
| 1 | 98 | 2:00
|
| 35 | 98 | 0:05
|
| 66 | 0:15
|
| 72 | 1:00
|
| 1 | 72 | 10:00
|
| 1 | 12 | inf
|
Amplification of fragment 2
A
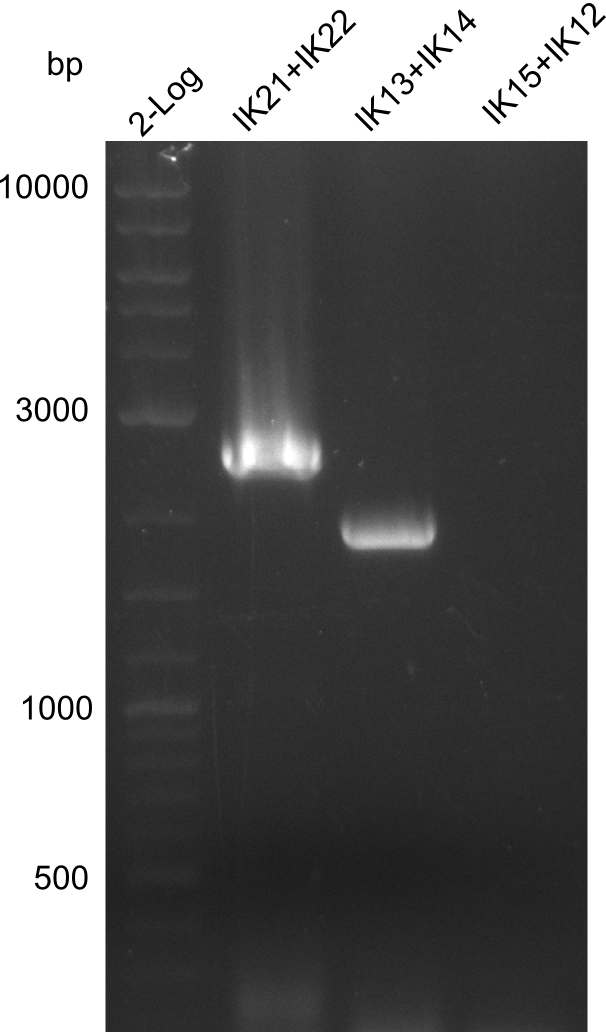
Lane 1: NEB 2-log; lane 2: pJM5 with primers IK21+IK22; lane 3:
B. parabrevis with primers IK13+IK14; lane 4:
B. parabrevis with primers IK15+IK12
| what | µl
|
| B. parabrevis | 1
|
| IK13 | 2
|
| IK14 | 2
|
| Q5 2x Master mix | 10
|
| ddH20 | 5
|
| Cycles | temperature [°C] | Time [s]
|
| 1 | 98 | 300
|
| 35 | 98 | 5
|
| 70 | 10
|
| 72 | 60
|
| 1 | 72 | 600
|
| 1 | 12 | inf
|
Result
Successful
B
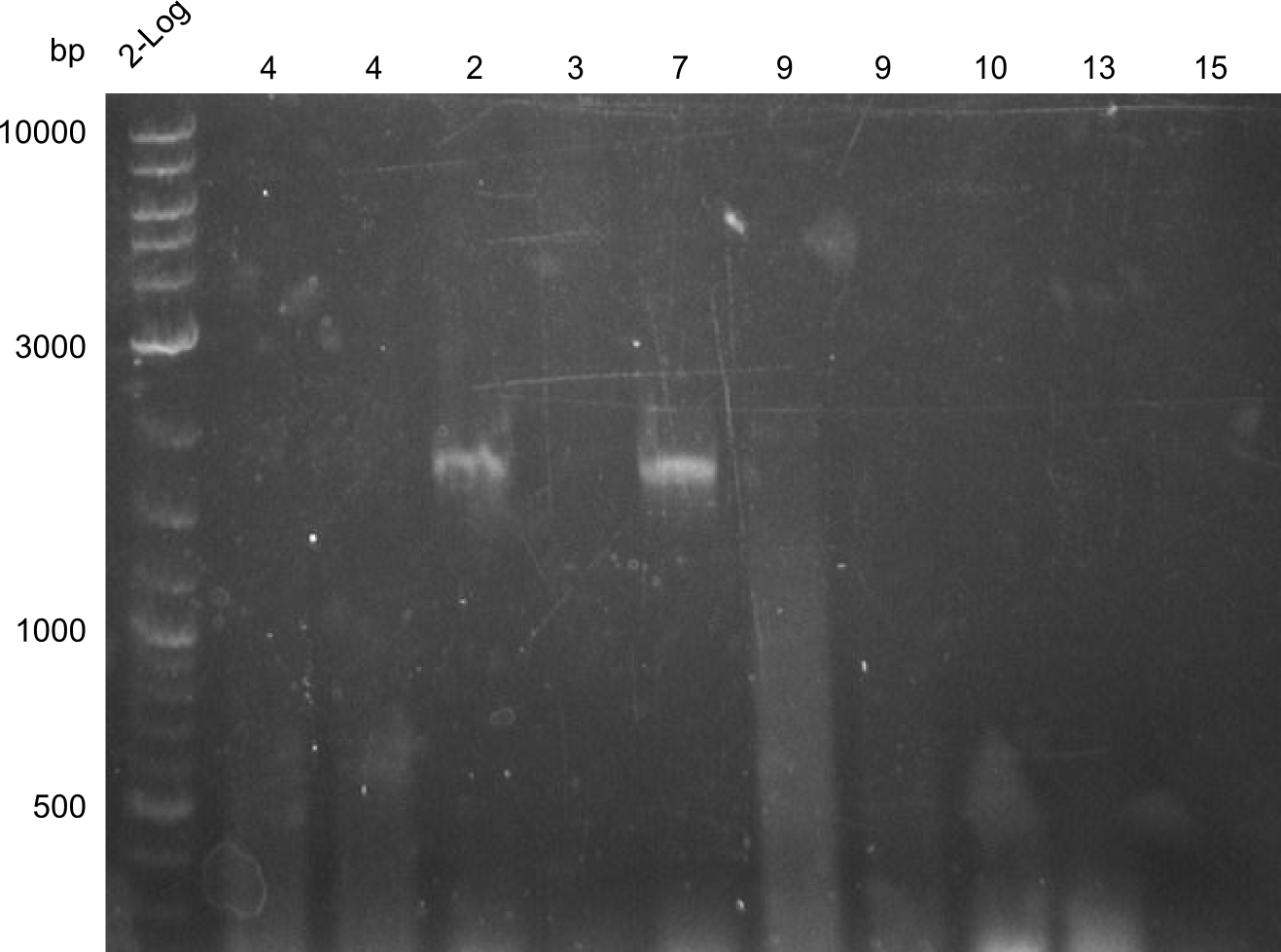
PCR Results of 27.07.2013 PCRs of fragments 2, 3, 4, 7, 9, 10, 13 & 15, fragment 2 and 7 were cut out for gel extraction
| what | µl
|
| Bacillus | 1
|
| IK13 | 2
|
| IK14 | 2
|
| Q5 2x Master mix | 10
|
| ddH20 | 5
|
| Cycles | temperature [°C] | Time [min:s]
|
| 1 | 98 | 2:00
|
| 35 | 98 | 0:05
|
| 70 | 0:15
|
| 72 | 1:00
|
| 1 | 72 | 10:00
|
| 1 | 12 | inf
|
PCR redone over night as gel was clumpy and did not show the expected fragments.
Amplification of fragment 3
A

Lane 1: NEB 2-log; lane 2: pJM5 with primers IK21+IK22; lane 3:
B. parabrevis with primers IK13+IK14; lane 4:
B. parabrevis with primers IK15+IK12
| what | µl
|
| B. parabrevis | 1
|
| IK12 | 2
|
| IK15 | 2
|
| Q5 2x Master mix | 10
|
| ddH20 | 5
|
| Cycles | temperature [°C] | Time [s]
|
| 1 | 98 | 300
|
| 35 | 98 | 5
|
| 66 | 10
|
| 72 | 120
|
| 1 | 72 | 600
|
| 1 | 12 | inf
|
Result
no band; new PCR settings
B
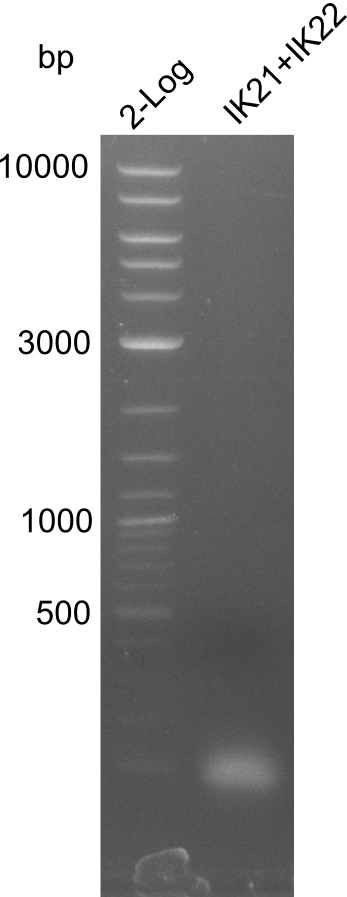
Lane 1: NEB 2-log; lane 2:
B. parabrevis with fragment 3
| Cycles | temperature [°C] | Time [s]
|
| 1 | 98 | 300
|
| 12 | 98 | 5
|
| 70 touchdown (-0.5°C) | 5
|
| 72 | 180
|
| 23 | 98 | 5
|
| 66 | 10
|
| 72 | 180
|
| 1 | 72 | 600
|
| 1 | 12 | inf
|
Result
xxxxxxxx; new: touchdown PCR with Phusion Flash
C

PCR Results of 25.07.13 2-log ladder and Tyr 3-8 with Q5
| what | µl
|
| primer fw | 2
|
| primer rv | 2
|
| DMSO | 1
|
| B. parabrevis | 1
|
| Phusion flash | 10
|
| ddH20 | 4
|
| Cycles | temperature [°C] | Time [s]
|
| 1 | 98 | 120
|
| 12 | 98 | 5
|
| 70 touchdown (-0.5°C) | 5
|
| 72 | 120
|
| 23 | 98 | 5
|
| 64 | 5
|
| 72 | 120
|
| 1 | 72 | 600
|
| 1 | 12 | inf
|
Result
Just a light band, have to improve conditions.
D
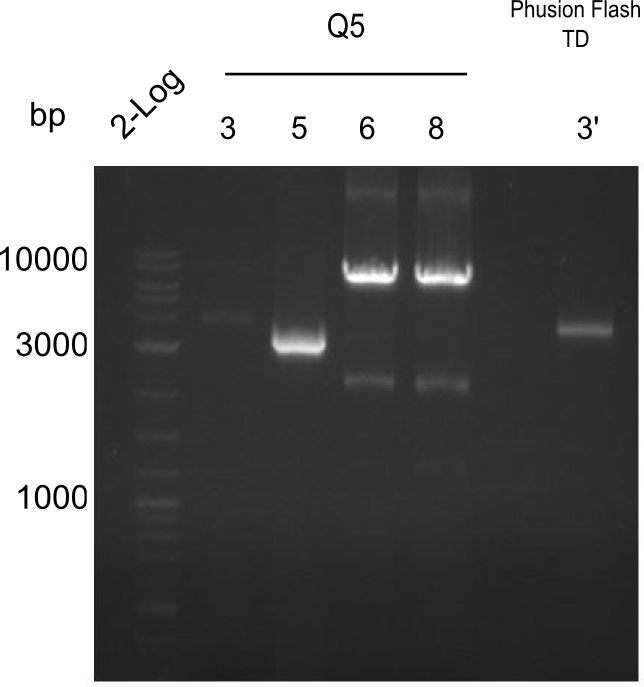
PCR Results of 26.07.13 Tyr 3,5,6,8 with Q5 and 3' with Phusion Flash. All were cut and extracted with Qiagen Gel Extraction Kit
Amplification with Q5
| what | µl
|
| Bacillus | 1
|
| IK12 | 2
|
| IK15 | 2
|
| Q5 2x Master mix | 10
|
| ddH20 | 5
|
| Cycles | temperature [°C] | Time [min:s]
|
| 1 | 98 | 2:00
|
| 35 | 98 | 0:05
|
| 66 | 0:15
|
| 72 | 2:00
|
| 1 | 72 | 10:00
|
| 1 | 12 | inf
|
E
Amplification with Phusion Flash
| what | µl
|
| Bacillus | 1
|
| IK12 | 2
|
| IK15 | 2
|
| Phusion 2x Master mix | 10
|
| DMSO | 1
|
| ddH20 | 4
|
| Cycles | temperature [°C] | Time [s]
|
| 1 | 98 | 2:00
|
| 12 | 98 | 0:05
|
| 70↓0.5°C | 0:05
|
| 72 | 2:30
|
| 23 | 98 | 0:05
|
| 64 | 0:05
|
| 72 | 3:00
|
| 1 | 72 | 10:00
|
| 1 | 12 | inf
|
F

PCR Results of 27.07.2013 PCRs of fragments 2, 3, 4, 7, 9, 10, 13 & 15, fragment 2 and 7 were cut out for gel extraction
| what | µl
|
| Bacillus | 1
|
| IK12 | 2
|
| IK15 | 2
|
| Q5 2x Master mix | 10
|
| ddH20 | 5
|
| Cycles | temperature [°C] | Time [min:s]
|
| 1 | 98 | 2:00
|
| 35 | 98 | 0:05
|
| 70 | 0:15
|
| 72 | 2:00
|
| 1 | 72 | 10:00
|
| 1 | 12 | inf
|
PCR redone over night as gel was clumpy and did not show the expected fragments.
Amplification of fragment 4
A

Lane 1: NEB 2-log; lane 2: pJM5 with primers IK21+IK22; lane 3:
B. parabrevis with primers IK13+IK14; lane 4:
B. parabrevis with primers IK15+IK12
| what | µl
|
| pSB1C3 | 0,5
|
| IK21 (1:10) | 2
|
| IK22 (1:10) | 2
|
| Q5 2x Master mix | 10
|
| ddH20 | 5,5
|
| Cycles | temperature [°C] | Time [s]
|
| 1 | 98 | 300
|
| 35 | 98 | 5
|
| 66 (fragment 4) | 10
|
| 72 | 120
|
| 1 | 72 | 600
|
| 1 | 12 | inf
|
Result
Successful
B

PCR Results of 27.07.2013 PCRs of fragments 2, 3, 4, 7, 9, 10, 13 & 15, fragment 2 and 7 were cut out for gel extraction
| what | µl
|
| pSB1C3 | 0.5
|
| IK21 | 2
|
| IK22 | 2
|
| Q5 2x Master mix | 10
|
| ddH20 | 5
|
| Cycles | temperature [°C] | Time [min:s]
|
| 1 | 98 | 2:00
|
| 35 | 98 | 0:05
|
| 66 | 0:15
|
| 72 | 1:00
|
| 1 | 72 | 10:00
|
| 1 | 12 | inf
|
Result
PCR redone over night as gel was clumpy and did not show the expected fragments.
C

all constructs extracted by gel extraction; fragment 15 missing -> Optimization
| what | µl
|
| IK21 | 2
|
| IK22 | 2
|
| pSB1C3 | 0.5
|
| Q5 2x Master mix | 10
|
| ddH20 | 5
|
| Cycles | temperature [°C] | Time [min:s]
|
| 1 | 98 | 2:00
|
| 35 | 98 | 0:05
|
| 66 | 0:15
|
| 72 | 1:00
|
| 1 | 72 | 10:00
|
| 1 | 12 | inf
|
Amplification of fragment 5
A

PCR Results of 25.07.13 2-log ladder and Tyr 3-8 with Q5
| what | µl
|
| B. Parabrevis | 1
|
| IK16 | 2
|
| IK17 | 2
|
| Q5 2x Master mix | 10
|
| ddH20 | 5,0
|
| Cycles | temperature [°C] | Time [s]
|
| 1 | 98 | 120
|
| 35 | 98 | 5
|
| 65 | 10
|
| 72 | 60
|
| 1 | 72 | 600
|
| 1 | 12 | inf
|
B

PCR Results of 26.07.13 Tyr 3,5,6,8 with Q5 and 3' with Phusion Flash. All were cut and extracted with Qiagen Gel Extraction Kit
| what | µl
|
| Bacillus | 1
|
| IK16 | 2
|
| IK17 | 2
|
| Q5 2x Master mix | 10
|
| ddH20 | 5
|
| Cycles | temperature [°C] | Time [min:s]
|
| 1 | 98 | 2:00
|
| 35 | 98 | 0:05
|
| 66 | 0:15
|
| 72 | 1:30
|
| 1 | 72 | 10:00
|
| 1 | 12 | inf
|
Amplification of fragment 6
A

PCR Results of 25.07.13 2-log ladder and Tyr 3-8 with Q5
| what | µl
|
| B. Parabrevis | 1
|
| IK12 | 2
|
| IK18 | 2
|
| Q5 2x Master mix | 10
|
| ddH20 | 5,0
|
| Cycles | temperature [°C] | Time [s]
|
| 1 | 98 | 120
|
| 35 | 98 | 5
|
| 66 | 10
|
| 72 | 150
|
| 1 | 72 | 600
|
| 1 | 12 | inf
|
B

PCR Results of 26.07.13 Tyr 3,5,6,8 with Q5 and 3' with Phusion Flash. All were cut and extracted with Qiagen Gel Extraction Kit
| what | µl
|
| Bacillus | 1
|
| IK12 | 2
|
| IK18 | 2
|
| Q5 2x Master mix | 10
|
| ddH20 | 5
|
| Cycles | temperature [°C] | Time [min:s]
|
| 1 | 98 | 2:00
|
| 35 | 98 | 0:05
|
| 66 | 0:15
|
| 72 | 3:00
|
| 1 | 72 | 10:00
|
| 1 | 12 | inf
|
Amplification of fragment 7
A

PCR Results of 25.07.13 2-log ladder and Tyr 3-8 with Q5
| what | µl
|
| B. Parabrevis | 1
|
| IK13 | 2
|
| IK19 | 2
|
| Q5 2x Master mix | 10
|
| ddH20 | 5,0
|
| Cycles | temperature [°C] | Time [s]
|
| 1 | 98 | 120
|
| 35 | 98 | 5
|
| 70 | 10
|
| 72 | 60
|
| 1 | 72 | 600
|
| 1 | 12 | inf
|
B

PCR Results of 27.07.2013 PCRs of fragments 2, 3, 4, 7, 9, 10, 13 & 15, fragment 2 and 7 were cut out for gel extraction
| what | µl
|
| Bacillus | 1
|
| IK13 | 2
|
| IK19 | 2
|
| Q5 2x Master mix | 10
|
| ddH20 | 5
|
| Cycles | temperature [°C] | Time [min:s]
|
| 1 | 98 | 2:00
|
| 35 | 98 | 0:05
|
| 70 | 0:15
|
| 72 | 1:00
|
| 1 | 72 | 10:00
|
| 1 | 12 | inf
|
PCR redone over night as gel was clumpy and did not show the expected fragments.
Amplification of fragment 8
A

PCR Results of 25.07.13 2-log ladder and Tyr 3-8 with Q5
| what | µl
|
| B. Parabrevis | 1
|
| IK20 | 2
|
| IK12 | 2
|
| Q5 2x Master mix | 10
|
| ddH20 | 5,0
|
| Cycles | temperature [°C] | Time [s]
|
| 1 | 98 | 120
|
| 35 | 98 | 5
|
| 66 | 10
|
| 72 | 150
|
| 1 | 72 | 600
|
| 1 | 12 | inf
|
B

PCR Results of 26.07.13 Tyr 3,5,6,8 with Q5 and 3' with Phusion Flash. All were cut and extracted with Qiagen Gel Extraction Kit
| what | µl
|
| Bacillus | 1
|
| IK12 | 2
|
| IK20 | 2
|
| Q5 2x Master mix | 10
|
| ddH20 | 5
|
| Cycles | temperature [°C] | Time [min:s]
|
| 1 | 98 | 2:00
|
| 35 | 98 | 0:05
|
| 66 | 0:15
|
| 72 | 3:00
|
| 1 | 72 | 10:00
|
| 1 | 12 | inf
|
Amplification of fragment 9
A

PCR Results of 27.07.2013 PCRs of fragments 2, 3, 4, 7, 9, 10, 13 & 15, fragment 2 and 7 were cut out for gel extraction
| what | µl
|
| pSB1C3 | 0.5
|
| PW04 | 2
|
| IK21 | 2
|
| Q5 2x Master mix | 10
|
| ddH20 | 5
|
| Cycles | temperature [°C] | Time [min:s]
|
| 1 | 98 | 2:00
|
| 35 | 98 | 0:05
|
| 66 | 0:15
|
| 72 | 1:00
|
| 1 | 72 | 10:00
|
| 1 | 12 | inf
|
PCR redone over night as gel was clumpy and did not show the expected fragments.
B

all constructs extracted by gel extraction; fragment 15 missing -> Optimization
| what | µl
|
| PW04 | 2
|
| IK22 | 2
|
| pSB1C3 | 0.5
|
| Q5 2x Master mix | 10
|
| ddH20 | 5
|
| Cycles | temperature [°C] | Time [min:s]
|
| 1 | 98 | 2:00
|
| 35 | 98 | 0:05
|
| 66 | 0:15
|
| 72 | 1:00
|
| 1 | 72 | 10:00
|
| 1 | 12 | inf
|
Amplification of fragment 10
A

PCR Results of 27.07.2013 PCRs of fragments 2, 3, 4, 7, 9, 10, 13 & 15, fragment 2 and 7 were cut out for gel extraction
| what | µl
|
| Bacillus | 1
|
| PW05 | 2
|
| PW06 | 2
|
| Q5 2x Master mix | 10
|
| ddH20 | 5
|
| Cycles | temperature [°C] | Time [min:s]
|
| 1 | 98 | 2:00
|
| 35 | 98 | 0:05
|
| 70 | 0:15
|
| 72 | 1:00
|
| 1 | 72 | 10:00
|
| 1 | 12 | inf
|
PCR redone over night as gel was clumpy and did not show the expected fragments.
B
| what | µl
|
| PW05 | 2
|
| PW06 | 2
|
| Bacillus | 1
|
| Q5 2x Master mix | 10
|
| ddH20 | 5
|
| Cycles | temperature [°C] | Time [min:s]
|
| 1 | 98 | 2:00
|
| 35 | 98 | 0:05
|
| 70 (fragment 10) | 0:15
|
| 72 | 1:00
|
| 1 | 72 | 10:00
|
| 1 | 12 | inf
|
Amplification of fragment 11
A
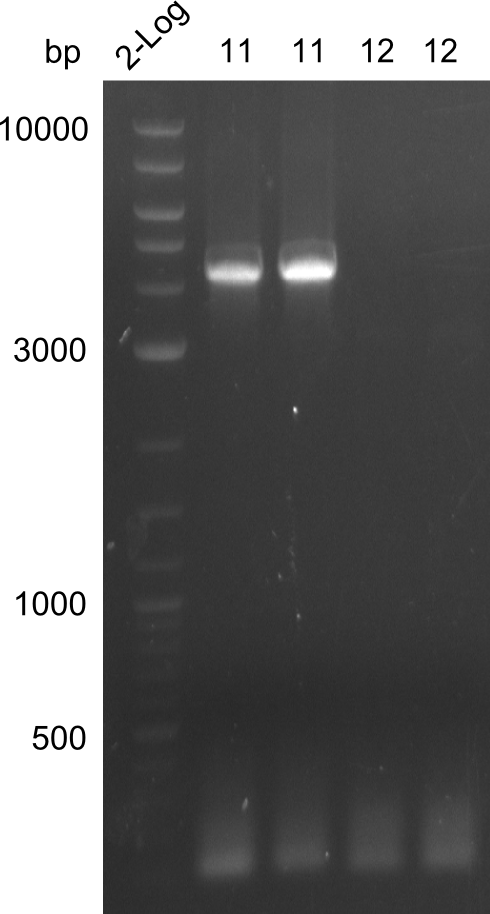
amplification of fragments 11 & 12. Fragment 11 is clearly visible, fragment 12 is missing
| what | µl
|
| PW07 | 2
|
| PW08 | 2
|
| Bacillus | 1
|
| Q5 2x Master mix | 10
|
| ddH20 | 5
|
| Cycles | temperature [°C] | Time [min:s]
|
| 1 | 98 | 2:00
|
| 35 | 98 | 0:05
|
| 72 | 0:15
|
| 72 | 2:30
|
| 1 | 72 | 10:00
|
| 1 | 12 | inf
|
Amplification of fragment 12
A

amplification of fragments 11 & 12. Fragment 11 is clearly visible, fragment 12 is missing
| what | µl
|
| PW09 | 2
|
| PW10 | 2
|
| Bacillus | 1
|
| Q5 2x Master mix | 10
|
| ddH20 | 5
|
| Cycles | temperature [°C] | Time [min:s]
|
| 1 | 98 | 2:00
|
| 35 | 98 | 0:05
|
| 69 | 0:15
|
| 72 | 1:30
|
| 1 | 72 | 10:00
|
| 1 | 12 | inf
|
Amplification of fragment 13
A

PCR Results of 27.07.2013 PCRs of fragments 2, 3, 4, 7, 9, 10, 13 & 15, fragment 2 and 7 were cut out for gel extraction
| what | µl
|
| Bacillus | 1 (for fragments 2, 3, 7, 10, 13 & 15)
|
| PW11 | 2
|
| IK12 | 2
|
| Q5 2x Master mix | 10
|
| ddH20 | 5
|
| Cycles | temperature [°C] | Time [min:s]
|
| 1 | 98 | 2:00
|
| 35 | 98 | 0:05
|
| 66 | 0:15
|
| 72 | 2:00
|
| 1 | 72 | 10:00
|
| 1 | 12 | inf
|
PCR redone over night as gel was clumpy and did not show the expected fragments.
B

all constructs extracted by gel extraction; fragment 15 missing -> Optimization
| what | µl
|
| PW11 | 2
|
| IK12 | 2
|
| Bacillus | 1
|
| Q5 2x Master mix | 10
|
| ddH20 | 5
|
| Cycles | temperature [°C] | Time [min:s]
|
| 1 | 98 | 2:00
|
| 35 | 98 | 0:05
|
| 66 | 0:15
|
| 72 | 2:00
|
| 1 | 72 | 10:00
|
| 1 | 12 | inf
|
Amplification of fragment 14
A

all constructs extracted by gel extraction; fragment 15 missing -> Optimization
| what | µl
|
| PW09 | 2
|
| IK12 | 2
|
| Bacillus | 1 (for fragments 2, 10, 13, 14 & 15)
|
| pSB1C3 | 0.5 (for fragments 1, 4 & 9)
|
| Q5 2x Master mix | 10
|
| ddH20 | 5
|
| Cycles | temperature [°C] | Time [min:s]
|
| 1 | 98 | 2:00
|
| 35 | 98 | 0:05
|
| 62 | 0:15
|
| 72 | 1:00
|
| 1 | 72 | 10:00
|
| 1 | 12 | inf
|
Amplification of fragment 15
A

PCR Results of 27.07.2013 PCRs of fragments 2, 3, 4, 7, 9, 10, 13 & 15, fragment 2 and 7 were cut out for gel extraction
| what | µl
|
| Bacillus | 1 (for fragments 2, 3, 7, 10, 13 & 15)
|
| PW13 | 2
|
| IK12 | 2
|
| Q5 2x Master mix | 10
|
| ddH20 | 5
|
| Cycles | temperature [°C] | Time [min:s]
|
| 1 | 98 | 2:00
|
| 35 | 98 | 0:05
|
| 66 | 0:15
|
| 72 | 2:00
|
| 1 | 72 | 10:00
|
| 1 | 12 | inf
|
PCR redone over night as gel was clumpy and did not show the expected fragments.
B
| what | µl
|
| PW13 | 2
|
| IK12 | 2
|
| Bacillus | 1
|
| Q5 2x Master mix | 10
|
| ddH20 | 5
|
| Cycles | temperature [°C] | Time [min:s]
|
| 1 | 98 | 2:00
|
| 35 | 98 | 0:05
|
| 66 | 0:15
|
| 72 | 2:00
|
| 1 | 72 | 10:00
|
| 1 | 12 | inf
|
Analysis of DNA concentrations of previously amplified fragments
Analytical Gel Electrophoresis 0.8% agarose
Scheme: each 1µL DNA (eluation in 20µL water)+ 3 µL loading dye
2log ladder, 4 µL 24.7:4, 25.7:1,2,3,7, 26.7.:3,5,6,8,3', 28.7.1,2,4,9,10,13,14 2log ladder, 4 µL

quantification gel of the fragments extracted from gel. See table for precise amounts
| fragment | concentration [ng/µl]
|
| 1 | 32
|
| 2 | 16
|
| 3 | --
|
| 4 | 30
|
| 5 | 12
|
| 6 | 8
|
| 7 | 12
|
| 8 | 8
|
| 9 | 30
|
| 10 | 40
|
| 11 | in progress
|
| 12 | in progress
|
| 13 | 4
|
| 14 | 40
|
| 15 | --
|
 "
"







