Team:Heidelberg/Tyrocidine week20 biobrickmod
From 2013.igem.org
Contents |
RFC10 Standardization of Tyc Modules
Sequencing
| Primer | OK? | Comments | File |
|---|---|---|---|
| Tyc AdCom | |||
| VR_rv | - | File:Heidelberg RFC10 TycASeq.zip | |
| VF2_fw | - | File:Heidelberg RFC10 TycASeq.zip | |
| Tyc B1 | |||
| VR_rv | silent mutations: 4595 GCC (Ala) to GCG (Ala); 4598 CTT (Leu) to CTG (Leu) | File:Heidelberg RFC10 TycB1Seq.zip | |
| VF2_fw | File:Heidelberg RFC10 TycB1Seq.zip | ||
| Tyc C5 | |||
| VR_rv | backbone was ok, wrong insert; since the insert was amplified with the same protocol like TycB1 we think that the primers were mixed up | File:Heidelberg RFC10 TycC5Seq.zip | |
| VF2_fw | backbone was ok, wrong insert; since the insert was amplified with the same protocol like TycB1 we think that the primers were mixed up | File:Heidelberg RFC10 TycC5Seq.zip | |
| Tyc C6 | |||
| VR_rv | File:Heidelberg RFC10 TycC6Seq.zip | ||
| VF2_fw | silent mutations: 4595 GTA (Val) to GTG (Val) | File:Heidelberg RFC10 TycC6Seq.zip | |
Tyrocidine-Indigoidine-fusion - standardization
So far, the Tyrocidine-Indigoidine-fusion constructs had two illegal cutting sites of RFC-10 restriction enzymes. Hence, we tried a CPEC-approach for which we reamplified a fragment from the vector with primers that introduced a mutation at the desired position.
Amplification
| what | µl |
|---|---|
| pPW05 (dil.) | 1 |
| RB68 | 2 |
| RB69 | 2 |
| Phusion Flash 2x Master Mix | 10 |
| ddH20 | 5 |
| Cycles | temperature [°C] | Time [min:s] |
|---|---|---|
| 1 | 98 | 0:05 |
| 35 | 98 | 0:05 |
| 59 | 0:10 | |
| 72 | 3:00 | |
| 1 | 72 | 10:00 |
| 1 | 10 | inf |
Results
CPEC
After gel-extraction of the large fragment, the following procedure was followed:
- 22µl H2O, 20µl of the large fragment, 2µl of the short fragment, 1µl DpnI and 5µl 10x CutSmart buffer were incubated for 2 hours at 37°C
- Mixture was purified with isoprop, washed with ethanol and eluted in 10µl H2O
- 10µl Phuson Flash Master Mix was added and the followin protocol was run:
| Cycles | temperature [°C] | Time [min:s] |
|---|---|---|
| 1 | 98 | 0:10 |
| 5 | 98 | 0:01 |
| 53 | 0:05 | |
| 72 | 3:00 | |
| 1 | 72 | 10:00 |
| 1 | 10 | inf |
Validation
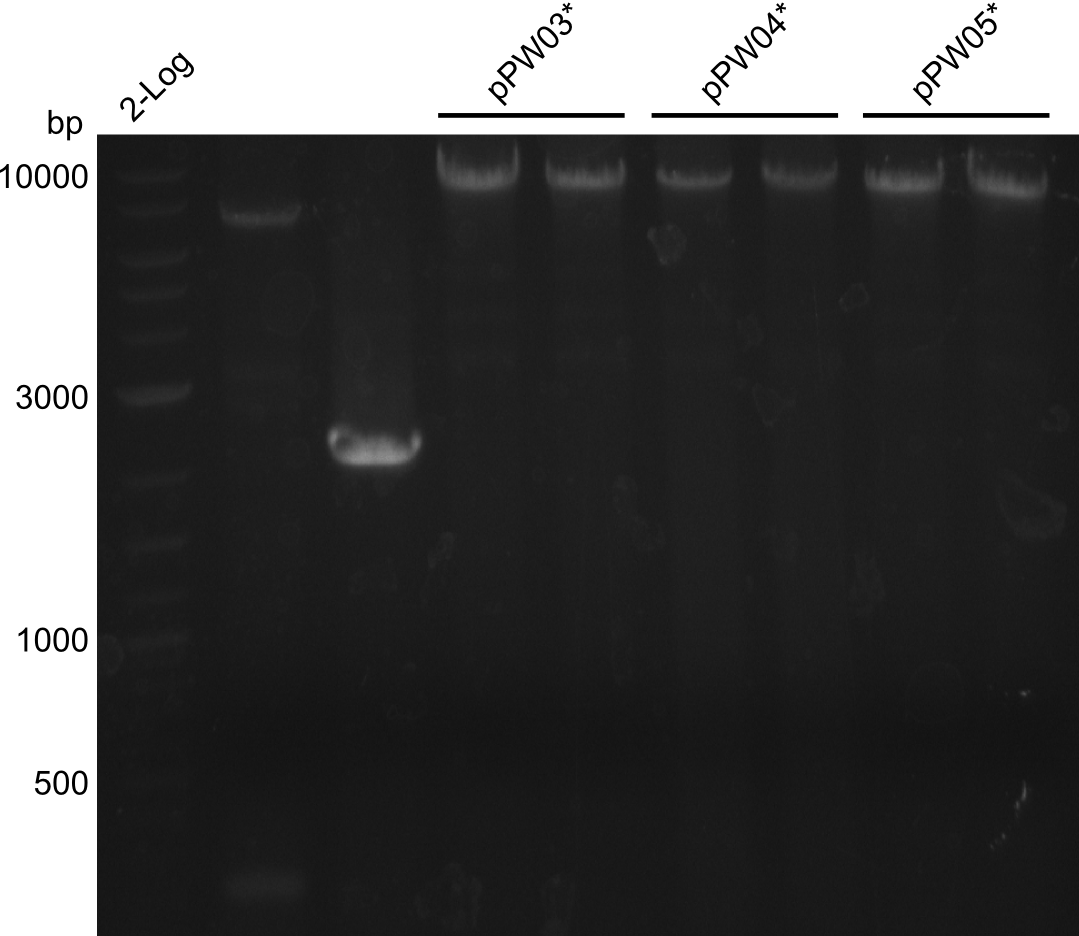
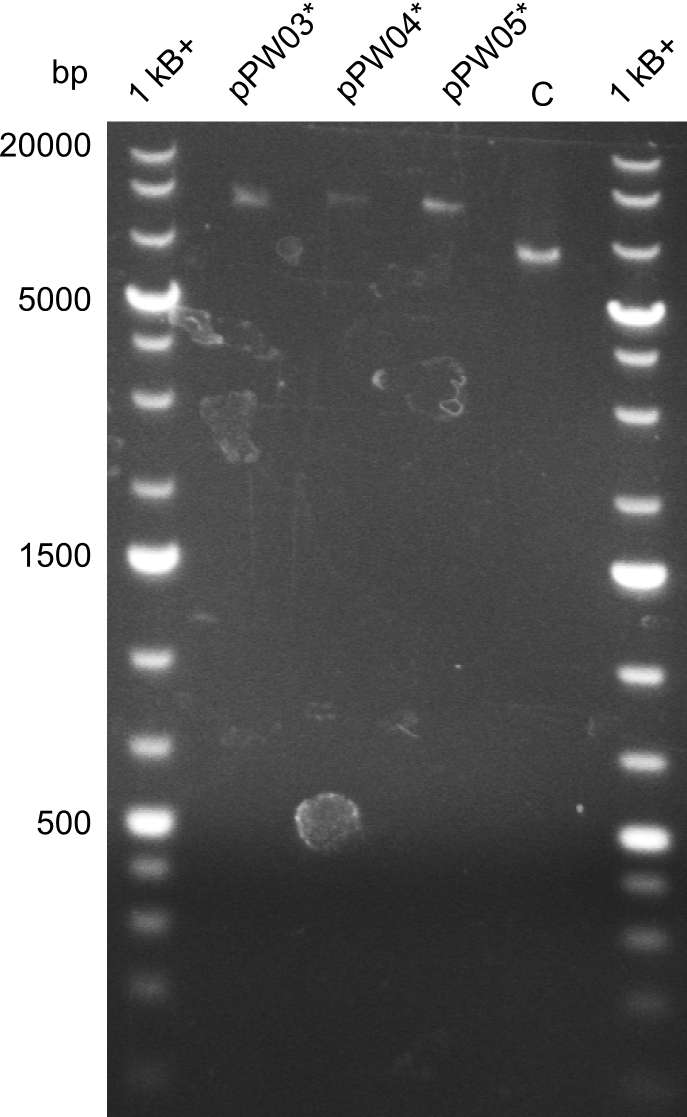
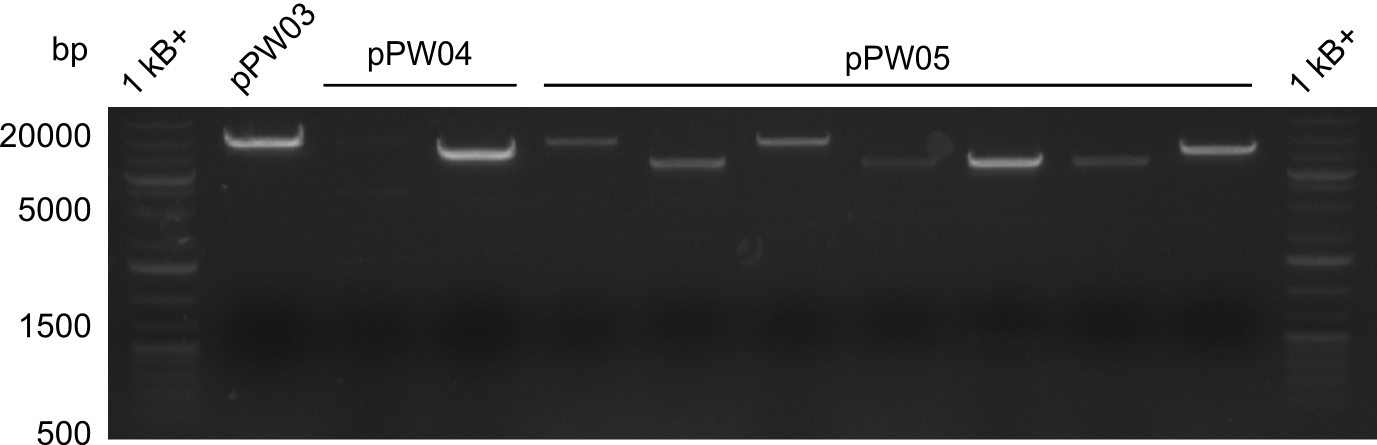
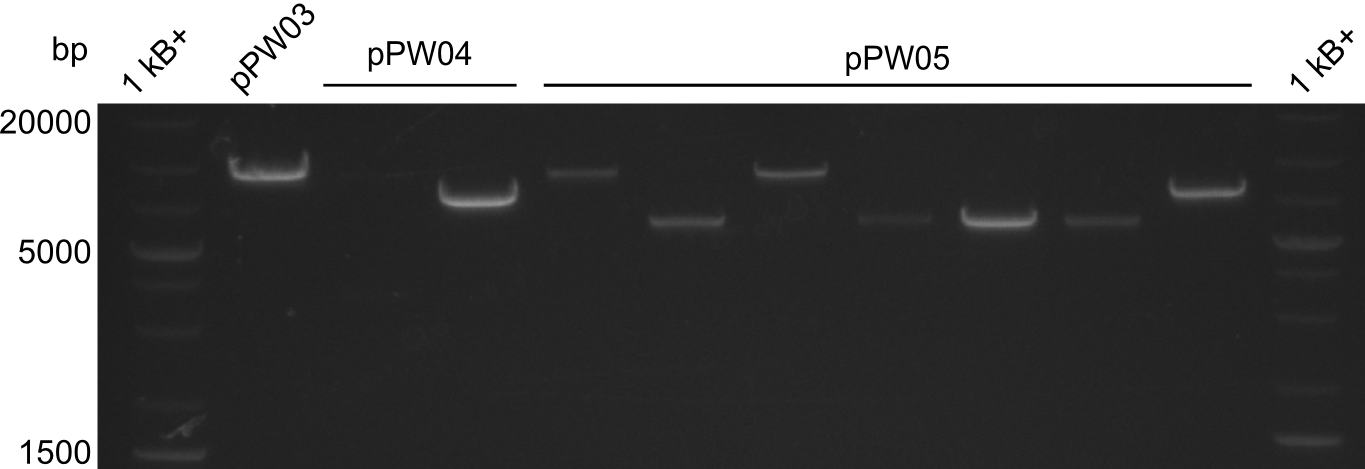
In order to validate colonies that were transformed with the CPEC-Mix, Restriction digest and sequencing was performed. As the unwanted restriction sites should have been mutated in the positive samples, using EcoRI as restriction enzyme should yield a single band at the size of the linearized vector (9049bp for pPW03, 9448bp for pPW04 and 8971bp for pPW05). Restriction digest was conducted with the standard protocol in a 20µl reaction volume.
The image on the lefthand-side shows the gel after 20 minutes at 100V, as this did not lead to good separation, the gel was let run for another 20 minutes. The gel after 40 minutes is displayed on the right side.
The sample for pPW01 and samples 1 and 3 for pPW05 were sent to sequencing as they migrated at the expected size.
Parallel-approach: Reassembly
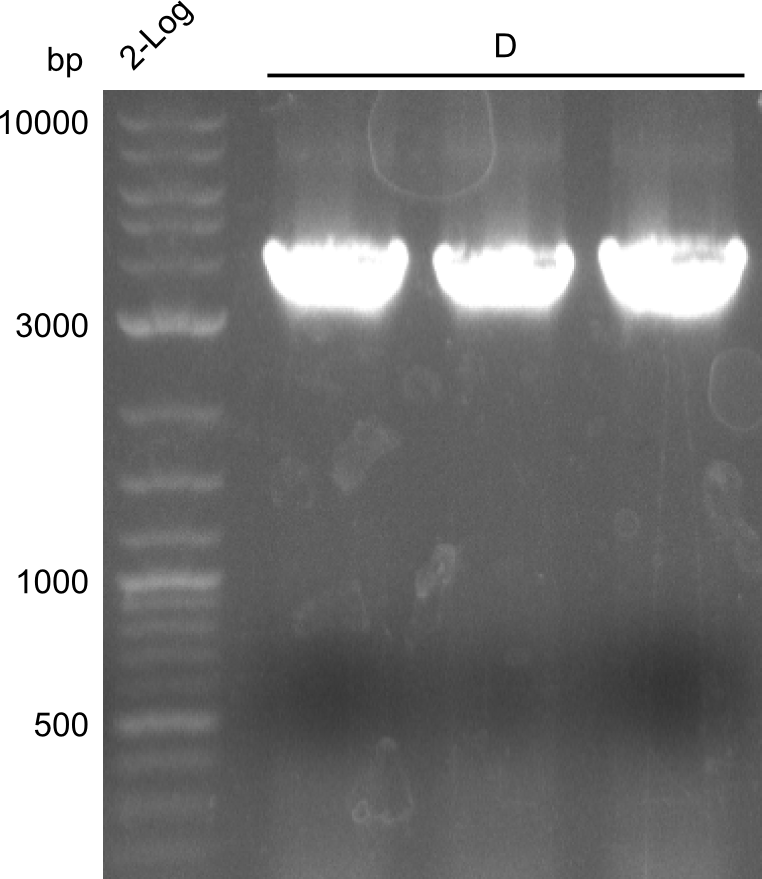
In order to have a second pillar to lean on, we decided to reamplify fragment D (indC) without the two restriction restriction sites. The template for this was created by the Indigoidine-group which had used the CPEC-approach described above.
| what | µl |
|---|---|
| indC* | 0.5 |
| PW15 | 2 |
| PW16 | 2 |
| Phusion Flash 2x Master mix | 10 |
| ddH20 | 5.5 |
| Cycles | temperature [°C] | Time [s] |
|---|---|---|
| 1 | 98 | 5 |
| 12 | 98 | 5 |
| 61 | 5 | |
| 72 | 100 | |
| 23 | 98 | 5 |
| 58 | 10 | |
| 72 | 100 | |
| 1 | 72 | 600 |
| 1 | 10 | inf |
We obtained (as expected) clearly visible, bright bands at the expected fragment size (~4000bp)
 "
"