|
|
| (7 intermediate revisions not shown) |
| Line 1: |
Line 1: |
| | {{TeamBYUProvo}} | | {{TeamBYUProvo}} |
| | | | |
| - | ==July==
| + | <br> |
| | | | |
| | + | {| width="100%" |
| | + | | colspan="3" | <font color="#333399" size="5" font face="Calibri"> |
| | | | |
| - | ===7/17/13===
| + | : '''Large Phage July - August Notebook: September 16 - September 27 Daily Log'''</font> |
| - | Results from 15 July 2013 -
| + | |
| - | We got our results from the titer test last time on the M9 and LB lysis inhibition test.
| + | |
| - | For the LB plates, we got 9 plaques on the 10^-10 plate, for a total of 9x10^12 pfus/mL. We were extremely excited about these results. The M9 was significantly less (we sort of expected this because we could not induce lysis as well with chloroform) with no plaques on the 10^-9. The spot tests confirmed these results (plaques on 10^-10 for the LB and plaques only down to 10^-6 for M9).
| + | |
| - | [[File:July15.1-1.jpg|400px]][[File:July15.1-2.jpg|400px]][[File:July15.1-3.jpg|400px]]
| + | |
| - | Today I did another round of mutagenesis using the lysis inhibition technique. I did two flasks. The first flask had 20 ug/mL of adenine which was added with the bacteria and 25 mL of LB broth; the second flask had 40 ug/mL of adenine added with the bacteria and 25 mL of LB broth. Both flasks incubated at 37C on the shaker for 3.5 hrs until slightly turbid. I then added 200 ug/mL of bromodeoxyuridine, 20 ug/mL of uracil, and .5 mL of phage to flask one; I added 400 ug/mL of bromodeoxyuridine, 40 ug/mL of uracil, and .5 mL of phage to flask two. They incubated in the same location for 3 hours.
| + | |
| | | | |
| - | After 3 hours, I pelleted the bacteria and processed them before (incubate with chloroform and nuclease, then pellet.) The purified lysates were slightly cloudy.
| + | <br> |
| | + | <br> |
| | | | |
| - | I poured the supernatants back into the original flasks and incubated them overnight to see if this was a possible way to produce a high-titer lysate of phage.
| + | |- valign="top" |
| | + | | style="width: 22%; background-color: transparent;"| |
| | | | |
| - | The purification group also centrifuged (in CsCl gradient) the T4 stock and the T4 LB mutagenesis / lysis inhibition sample. After dialysis, they will return individual fractions to us.
| + | <font color="#333399" size="3" font face="Calibri"> |
| | | | |
| - | ===7/19/13=== | + | <font size = "4"> |
| - | Today I processed the flasks I had let incubate overnight beginning with just the phage supernatant. After 24 hours, the flasks were completely turbid. After processing, the high titer lysates were not as turbid as the previous ones, showing this is not an ideal way to grow large concentrations of phage.
| + | |
| | | | |
| - | I also received the pictures from the phage purification group of the cesium chloride gradient results. The mutagenized phage stock showed a clear (but slightly denser) normal size band, a faint petite band, and no band where large phage should be. They were separated into five fractions (two petite, two normal, and 1 large).
| + | : <u> '''Large Phage''' </u> </font> |
| - | [[File:CesiumChloride1.jpg|400px]]
| + | |
| - | [[File:CesiumChloride2.jpg|400px]]
| + | |
| - | [[File:CesiumChloride3.jpg|400px]]
| + | |
| - | [[File:CesiumChloride4.jpg|400px]]
| + | |
| - | ===7/22/13===
| + | |
| - | Today we discussed plans for our children's book and community outreach. We edited the pre and post test.
| + | |
| - | The purification group did the dialysis on the samples. When they are done, we will titer the five fractions.
| + | |
| | | | |
| - | I also typed the following procedure for the small phage group to help them in their mutagenesis.
| + | : [[Team:BYU Provo/Notebook/LargePhage/Winterexp|March-April]] |
| | | | |
| - | Mutagenesis experiment:
| + | : [[Team:BYU Provo/Notebook/LargePhage/Springexp|May-June]] |
| - | In a 250 mL flask, add 25 mL LB broth, 20 ug/mL adenine, and 1 loop of E. coli bacteria. Let incubate on shaker at 37C for ~2 hours until moderately turbid.
| + | |
| - | At this point, you can pellet the bacteria and resuspend it in minimal (M9) media or leave it in the LB broth (unpelleted).
| + | |
| - | At this point, add another 20 ug/mL of adenine, 20 ug/mL of uracil, 200 ug/mL of bromodeoxyuridine (mutagen), and the appropriate amount of phage (we use 1x10^9 pfu) for the bacterial concentration you have. Let incubate on shaker at 37C for 3 hours.
| + | |
| | | | |
| - | After 3 hours, transfer 5 mL of the culture from the flask to a capped test tube. Add 1 mL of chloroform, shake gently, and let it sit for 5 min. If it clears, the culture is ready to harvest.
| + | : [[Team:BYU Provo/Notebook/LargePhage/Summerexp|July-August]] |
| - | Pellet the bacteria/phage culture from the flask at 3500 rpm, and resuspend the pellet it in 2 mL of LB broth. Large phage group: discard the supernatant. Small phage group: keep the supernatant and compare the titer of the supernatant with the lysed pellet (follow the following instructions.
| + | |
| | | | |
| - | Transfer resuspended pellet to a capped test tube, add 3 mL of chloroform, 10 uL of 1 M MgCl2, and 40 uL of nuclease. Shake gently and incubate at room temp for 30 mins.
| + | : [[Team:BYU Provo/Notebook/LargePhage/Fallexp|September-October]] |
| - | Pellet at 2700 rpm for 10 minutes, then transfer the supernatant to a new test tube. This is your high titer lysate.
| + | |
| | | | |
| - | ===7/24/13===
| + | </font> |
| | | | |
| - | ===7/26/13=== | + | | style="width: 82%; background-color: transparent;"| |
| | | | |
| - | ===7/29/13=== | + | <font face="Calibri" size="3"> |
| | | | |
| - | ===7/31/13=== | + | <font size="4"> '''9/16/13''' </font> |
| - | Today we ran spot test on the -4 and -5 plates of the purified phage.
| + | |
| - | [[File:July29-1.jpg|400px]]
| + | |
| - | [[File:July29-2.jpg|400px]]
| + | |
| - | [[File:July29-3.jpg|400px]]
| + | |
| - | [[File:July29-4.jpg|400px]]
| + | |
| - | [[File:July29-5.jpg|400px]]
| + | |
| - | [[File:July29-6.jpg|400px]]
| + | |
| - | [[File:July29-7.jpg|400px]]
| + | |
| - | [[File:July29-8.jpg|400px]]
| + | |
| | | | |
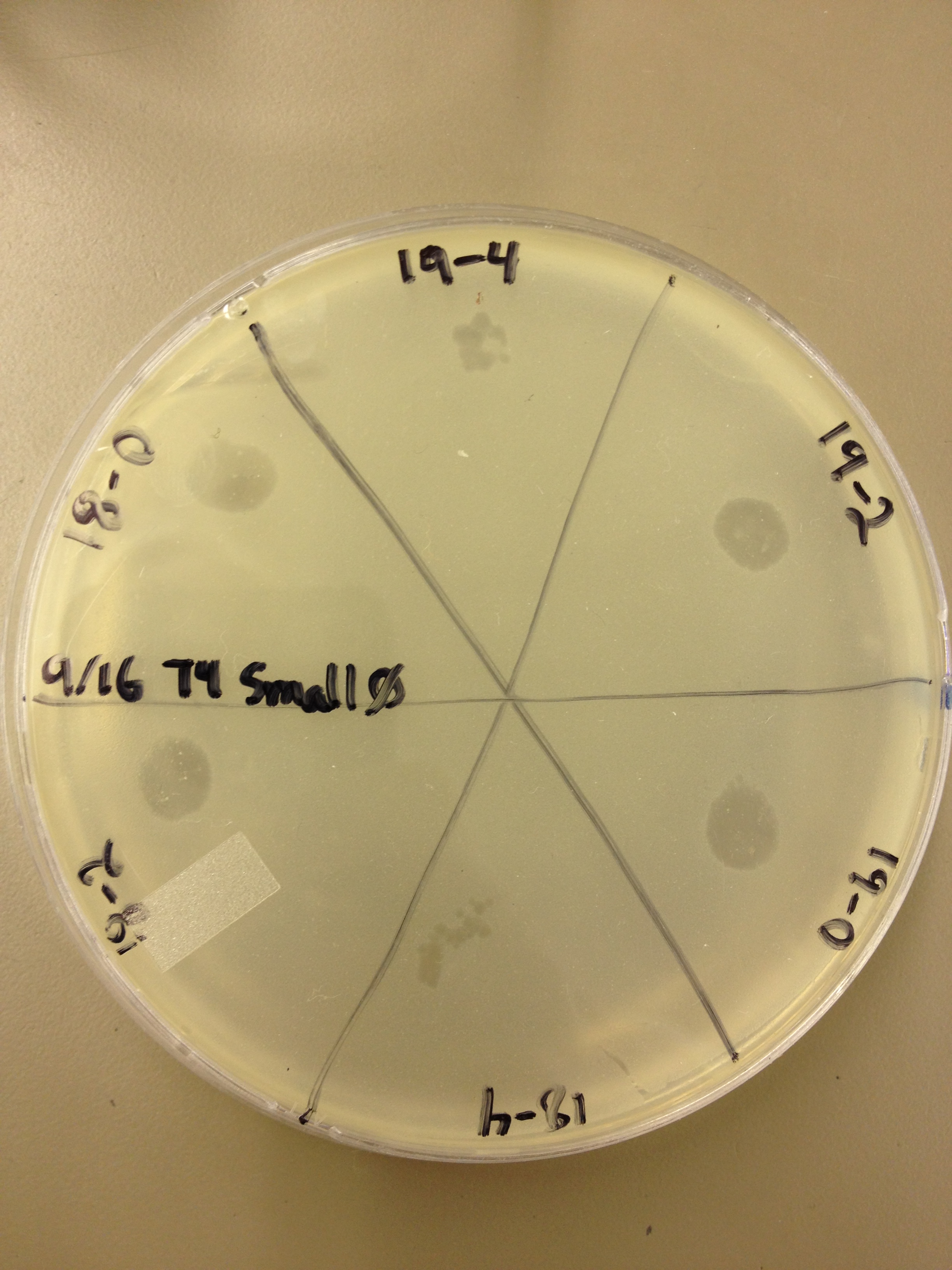
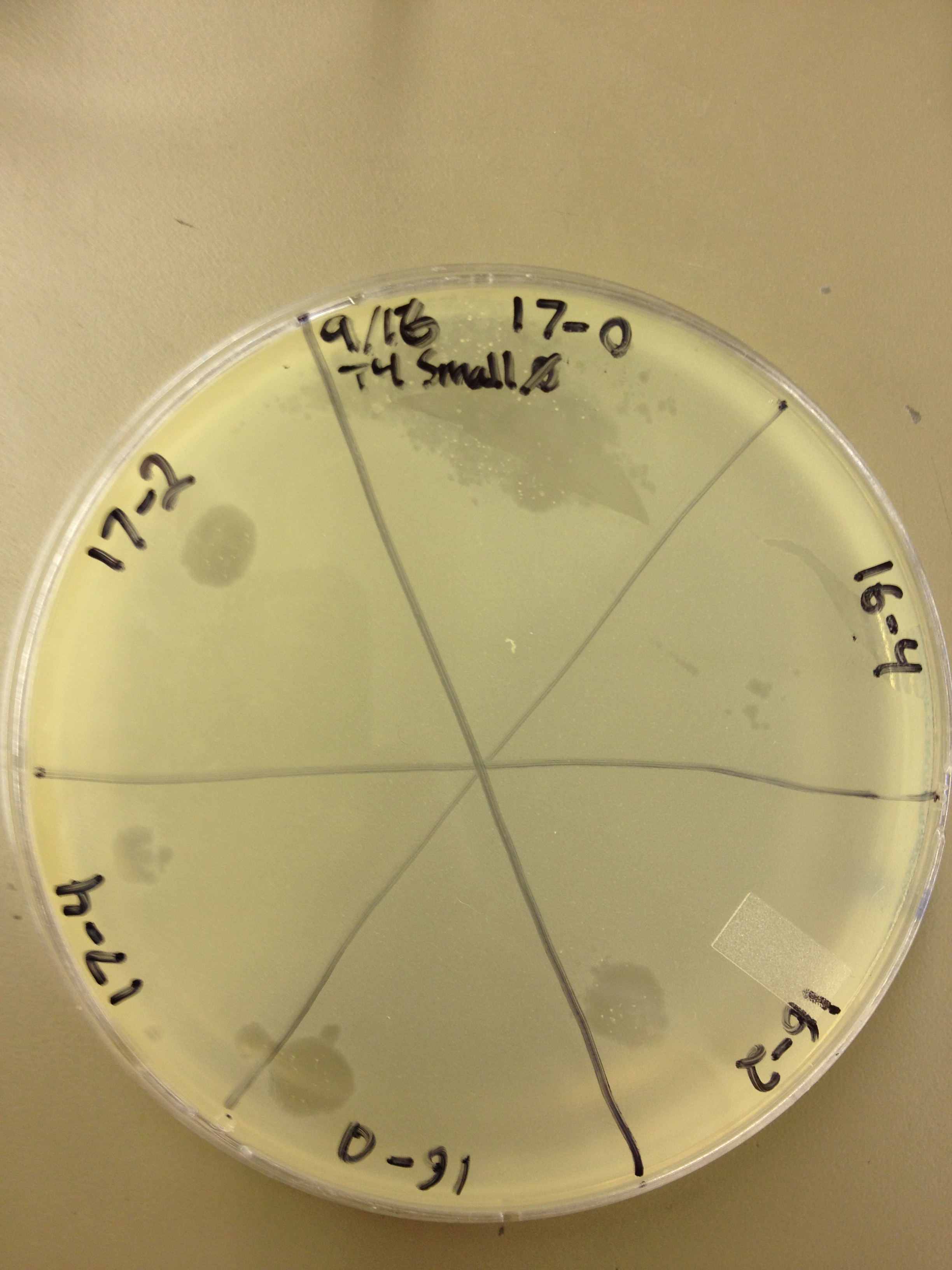
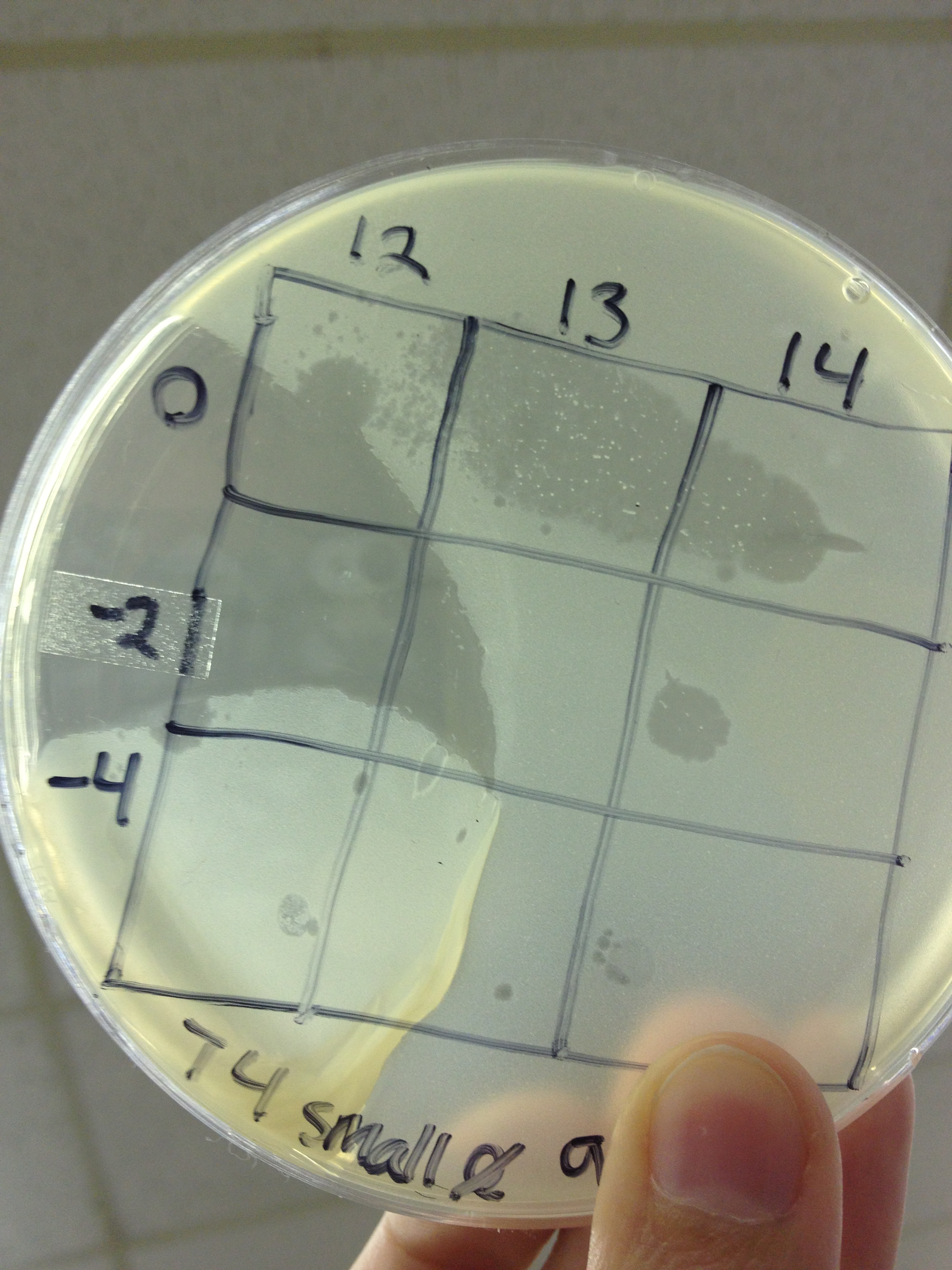
| - | {{TeamBYUProvoFooter}}0 | + | Today Jade and LJ helped us spot test all of the fractions above the band from our last CsCl gradient. These fractions were numbered 12-19, but 15 was skipped. Each fraction was spot tested at a titer of 10^0, 10^-2, and 10^-4 and incubated agar side down at 37C overnight. |
| | + | |
| | + | <br> |
| | + | |
| | + | <font size="4"> '''9/18/13''' </font> |
| | + | |
| | + | We looked at the results from the spot test today. We found that: |
| | + | All fractions had phage at 10^0 |
| | + | Fractions 18, 19, 17, 16, and 14 had large clearings still at 10^-2 while 13 and 12 just had individual plaques. |
| | + | Fractions 18, 19, 17, 16, and 14 had individual plaques at 10^-4. |
| | + | |
| | + | We also measured the capsids and tails of the TEM images. |
| | + | |
| | + | |
| | + | [[File:September18-1.jpg|400px]] |
| | + | |
| | + | [[File:September18-2.jpg|400px]] |
| | + | |
| | + | [[File:September18-3.jpg|400px]] |
| | + | |
| | + | <br> |
| | + | |
| | + | <font size="4"> '''9/23/13''' </font> |
| | + | |
| | + | We spent our time in class and over the weekend measuring the phage capsids and tails to see if there is a correlation between their sizes before and after our mutagenesis. |
| | + | |
| | + | <br> |
| | + | |
| | + | <font size="4"> '''9/25/13''' </font> |
| | + | |
| | + | -Today we worked on the project poster image for the phage diagram |
| | + | |
| | + | <br> |
| | + | |
| | + | |
| | + | {{TeamBYUProvoFooter}} |
 "
"