Team:BYU Provo/Notebook/LargePhage/Summerexp/Period4/Dailylog
From 2013.igem.org
(→8/20/13) |
|||
| Line 1: | Line 1: | ||
{{TeamBYUProvo}} | {{TeamBYUProvo}} | ||
| - | ==August== | + | <br> |
| - | === | + | |
| + | {| width="100%" | ||
| + | | colspan="3" | <font color="#333399" size="5" font face="Calibri"> | ||
| + | |||
| + | : '''Large Phage July - August Notebook: August 15 - August 31 Daily Log'''</font> | ||
| + | |||
| + | <br> | ||
| + | <br> | ||
| + | |||
| + | |- valign="top" | ||
| + | | style="width: 18%; background-color: transparent;"| | ||
| + | |||
| + | <font color="#333399" size="3" font face="Calibri"> | ||
| + | |||
| + | <font size = "4"> | ||
| + | |||
| + | : <u> '''Small Phage''' </u> </font> | ||
| + | |||
| + | : [[Team:BYU Provo/Notebook/LargePhage/Winterexp|March-April]] | ||
| + | |||
| + | : [[Team:BYU Provo/Notebook/LargePhage/Springexp|May-June]] | ||
| + | |||
| + | : [[Team:BYU Provo/Notebook/LargePhage/Summerexp|July-August]] | ||
| + | |||
| + | : [[Team:BYU Provo/Notebook/LargePhage/Fallexp|September-October]] | ||
| + | |||
| + | </font> | ||
| + | |||
| + | | style="width: 82%; background-color: transparent;"| | ||
| + | |||
| + | <font face="Calibri" size="3"> | ||
| + | |||
| + | <font size="4"> '''8/17/13''' </font> | ||
[[File:Aug16-1.jpg|400px]] | [[File:Aug16-1.jpg|400px]] | ||
[[File:Aug16-2.jpg|400px]] | [[File:Aug16-2.jpg|400px]] | ||
| Line 10: | Line 42: | ||
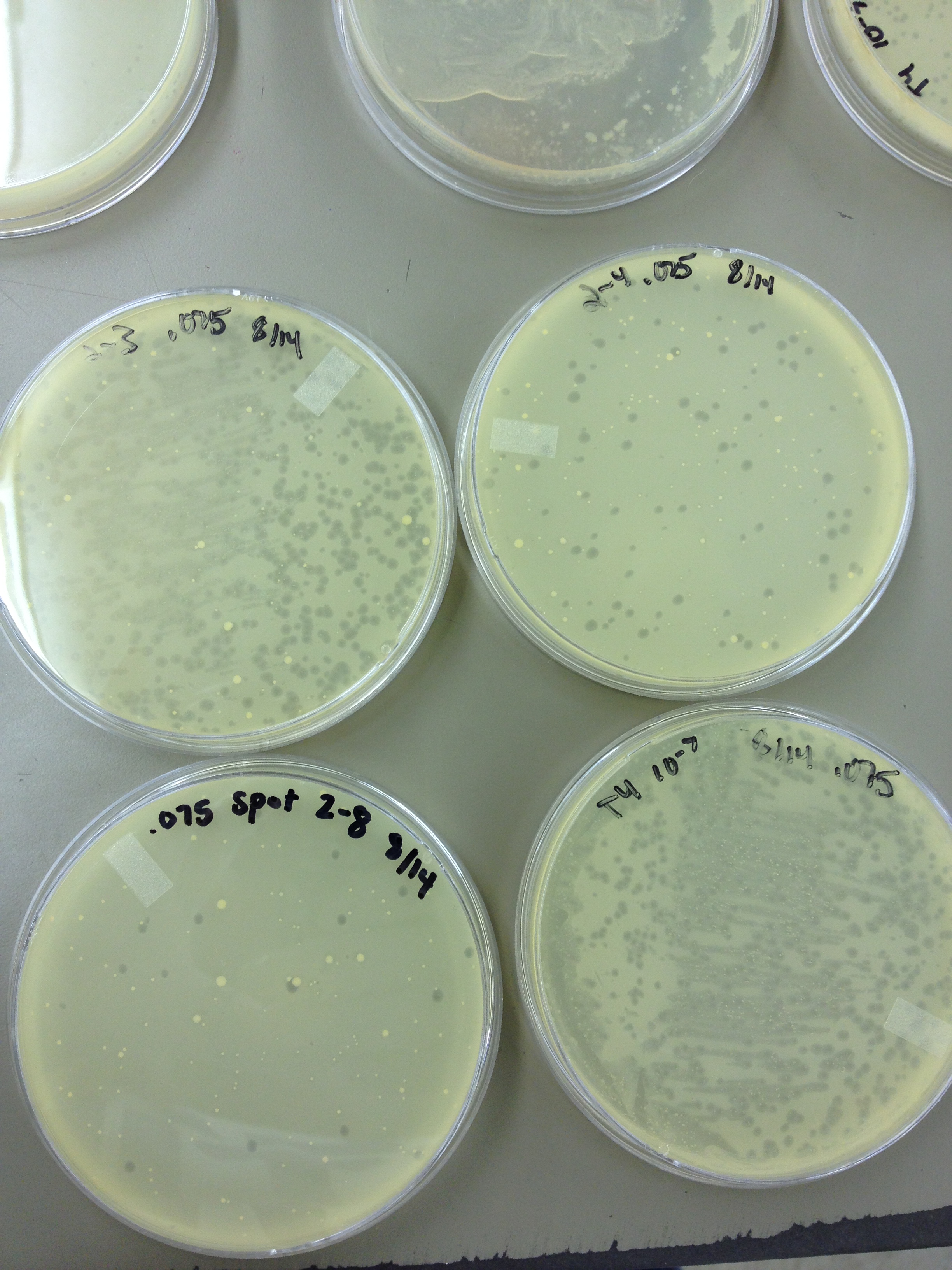
We also did a spot test to verify that there was/ was not phage in the lower and higher dilutions as we suspected. | We also did a spot test to verify that there was/ was not phage in the lower and higher dilutions as we suspected. | ||
| - | = | + | <font size="4"> '''8/20/13''' </font> |
[[File:August19.1.jpg|400px|center]] | [[File:August19.1.jpg|400px|center]] | ||
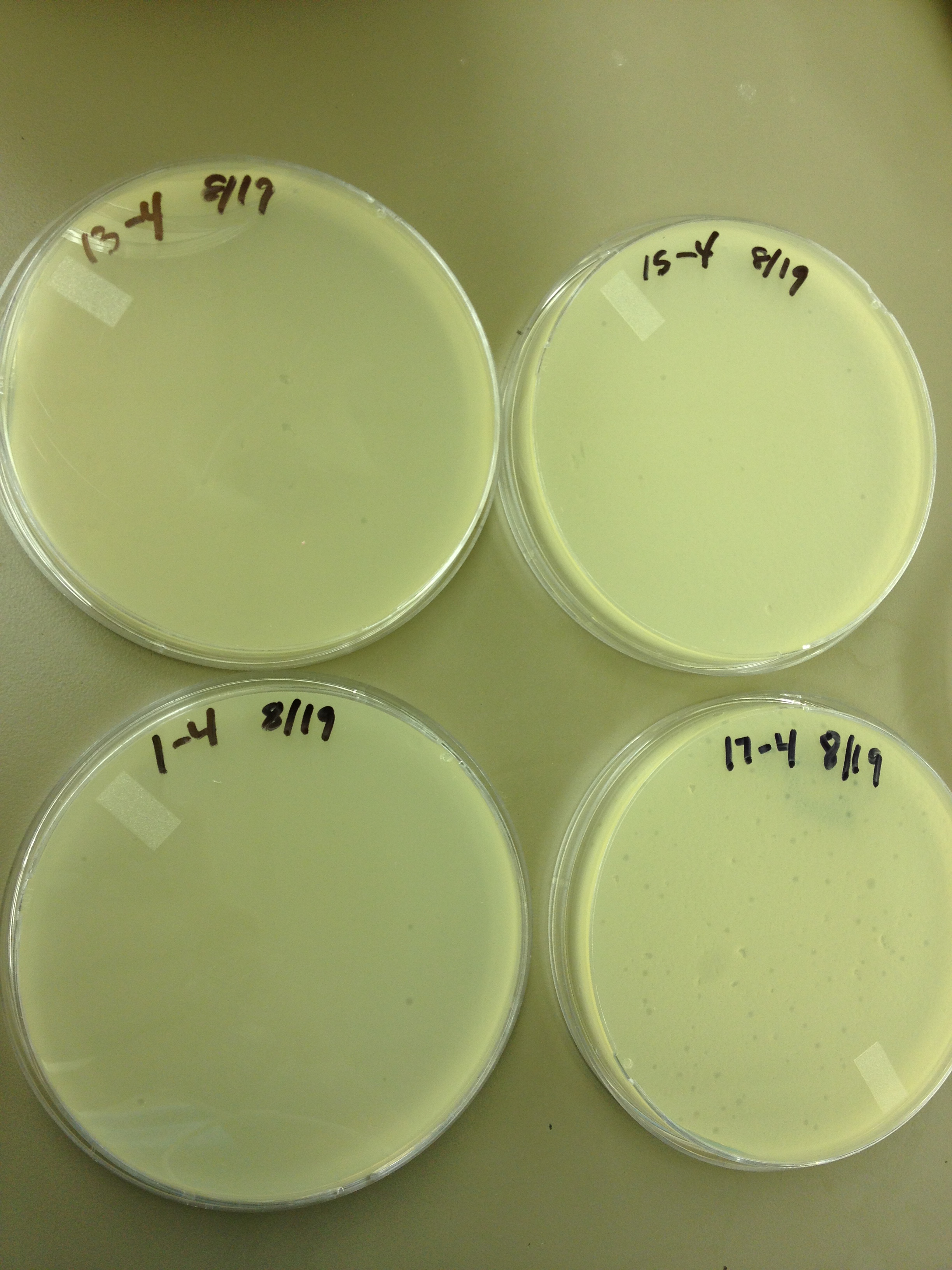
| Line 31: | Line 63: | ||
Plate 17: 10^-4 | Plate 17: 10^-4 | ||
| - | = | + | <font size="4"> '''8/21/13''' </font> |
Today we started a new mutagenesis experiment. The Protocol being: | Today we started a new mutagenesis experiment. The Protocol being: | ||
In a 250 mL flask, add 25 mL LB broth, 20 ug/mL adenine, and 1 loop of E. coli bacteria. Let incubate on shaker at 37C for ~2 hours until moderately turbid. At this point, you can pellet the bacteria and resuspend it in minimal (M9) media or leave it in the LB broth (unpelleted). At this point, add another 20 ug/mL of adenine, 20 ug/mL of uracil, 200 ug/mL of bromodeoxyuridine (mutagen), and the appropriate amount of phage (we use 1x10^9 pfu) for the bacterial concentration you have. Let incubate on shaker at 37C for 3 hours. | In a 250 mL flask, add 25 mL LB broth, 20 ug/mL adenine, and 1 loop of E. coli bacteria. Let incubate on shaker at 37C for ~2 hours until moderately turbid. At this point, you can pellet the bacteria and resuspend it in minimal (M9) media or leave it in the LB broth (unpelleted). At this point, add another 20 ug/mL of adenine, 20 ug/mL of uracil, 200 ug/mL of bromodeoxyuridine (mutagen), and the appropriate amount of phage (we use 1x10^9 pfu) for the bacterial concentration you have. Let incubate on shaker at 37C for 3 hours. | ||
| Line 39: | Line 71: | ||
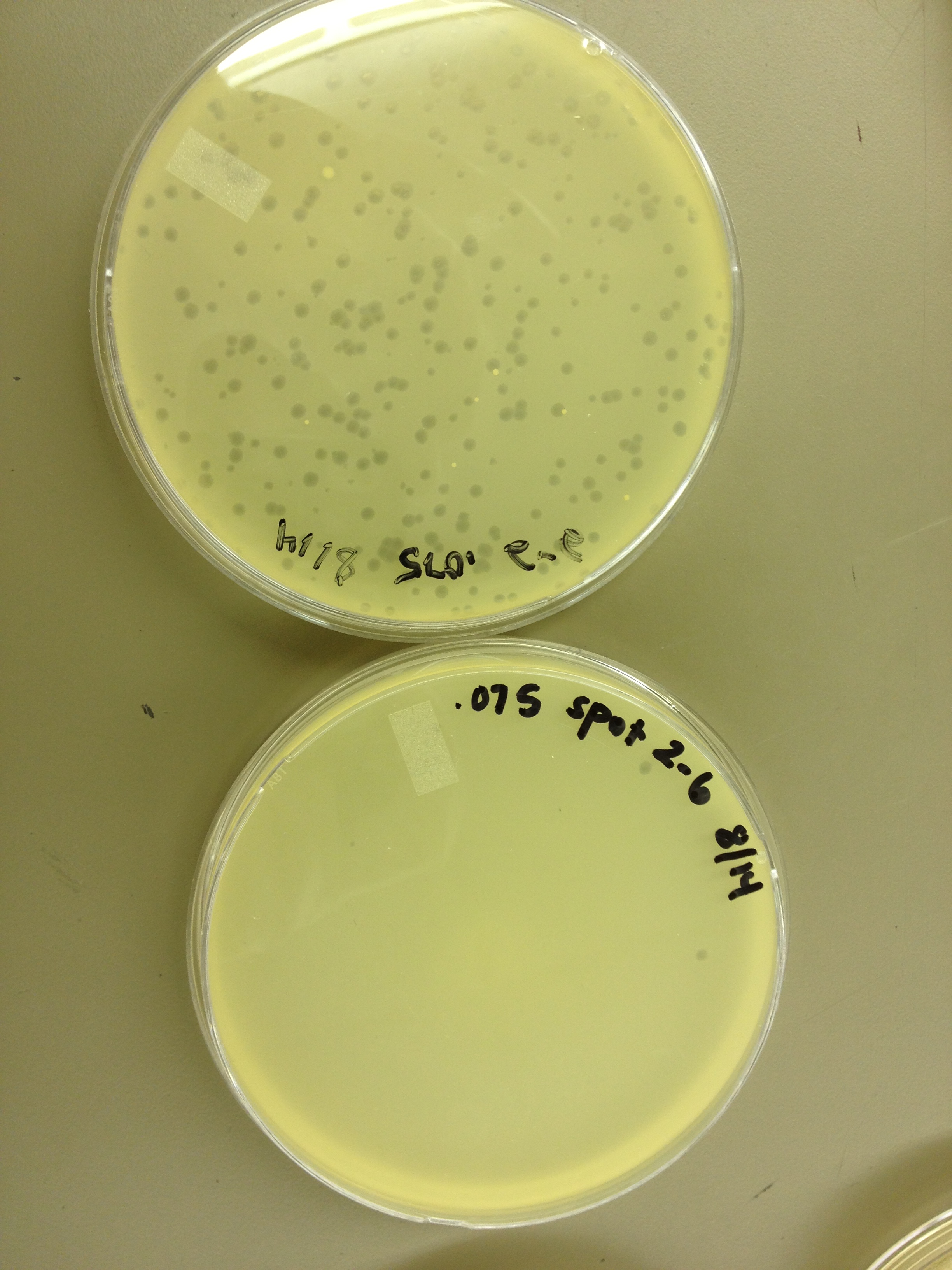
Today we are also doing a spot test as an initial test for the even numbered fractions from the gradient. | Today we are also doing a spot test as an initial test for the even numbered fractions from the gradient. | ||
| - | = | + | <font size="4"> '''8/28/13''' </font> |
Today we got our mutagenesis back from the purification group. It came in 21 fractions. Fractions 1-9 were from below the band, 10-12 were where the band was, 13-19 were above the band, and 20-21 were where the bacterial debris was. I did a spot test of fractions 1-6 and 20-21 at 10^0 and 10^-2 (5 uL of each on a plate). | Today we got our mutagenesis back from the purification group. It came in 21 fractions. Fractions 1-9 were from below the band, 10-12 were where the band was, 13-19 were above the band, and 20-21 were where the bacterial debris was. I did a spot test of fractions 1-6 and 20-21 at 10^0 and 10^-2 (5 uL of each on a plate). | ||
{{TeamBYUProvoFooter}} | {{TeamBYUProvoFooter}} | ||
Revision as of 21:42, 26 September 2013
| ||
|
|
8/19/13Today we started to do titers on the .5 mL fractions that were taken from the Cesium Chloride gradient. In order to get a general idea, and save some time, we did every other fraction. We also did a spot test to verify that there was/ was not phage in the lower and higher dilutions as we suspected. 8/20/13
Today started the dilution series for the other half of the fractions from the cesium chloride gradient, so it will be ready when we want to plate them out. From the spot test and plating of the dilution series, the fractions look like this: Plate 1: 10^-4 Plate 3: 10^-2 Plate 5: 10^-2 Plate 7: 10^-2 Plate 9: 10^-2 Plate 11: 10^-2 Plate 13: 10^-4 Plate 15: 10^-4 Plate 17: 10^-4 8/21/13 Today we started a new mutagenesis experiment. The Protocol being: In a 250 mL flask, add 25 mL LB broth, 20 ug/mL adenine, and 1 loop of E. coli bacteria. Let incubate on shaker at 37C for ~2 hours until moderately turbid. At this point, you can pellet the bacteria and resuspend it in minimal (M9) media or leave it in the LB broth (unpelleted). At this point, add another 20 ug/mL of adenine, 20 ug/mL of uracil, 200 ug/mL of bromodeoxyuridine (mutagen), and the appropriate amount of phage (we use 1x10^9 pfu) for the bacterial concentration you have. Let incubate on shaker at 37C for 3 hours. After 3 hours, transfer 5 mL of the culture from the flask to a capped test tube. Add 1 mL of chloroform, shake gently, and let it sit for 5 min. If it clears, the culture is ready to harvest. Pellet the bacteria/phage culture from the flask at 3500 rpm, and resuspend the pellet it in 2 mL of LB broth. Large phage group: discard the supernatant. Small phage group: keep the supernatant and compare the titer of the supernatant with the lysed pellet (follow the following instructions. Transfer resuspended pellet to a capped test tube, add 3 mL of chloroform, 10 uL of 1 M MgCl2, and 40 uL of nuclease. Shake gently and incubate at room temp for 30 mins. Pellet at 2700 rpm for 10 minutes, then transfer the supernatant to a new test tube. This is your high titer lysate. Today we are also doing a spot test as an initial test for the even numbered fractions from the gradient. 8/28/13 Today we got our mutagenesis back from the purification group. It came in 21 fractions. Fractions 1-9 were from below the band, 10-12 were where the band was, 13-19 were above the band, and 20-21 were where the bacterial debris was. I did a spot test of fractions 1-6 and 20-21 at 10^0 and 10^-2 (5 uL of each on a plate).
|
|
 "
"