Team:Hong Kong HKU/humanpractice/futureapps
From 2013.igem.org
(Difference between revisions)
SamsonWlsh (Talk | contribs) |
|||
| (10 intermediate revisions not shown) | |||
| Line 72: | Line 72: | ||
<div id="content1"> | <div id="content1"> | ||
<font face="Impact" size="7" color="green"> | <font face="Impact" size="7" color="green"> | ||
| - | Future | + | Future Applications<br><br> |
</font> | </font> | ||
<font face="impact" size="5" color="green"> | <font face="impact" size="5" color="green"> | ||
| - | + | Revisit to Our Targeted Problem<br><br> | |
</font> | </font> | ||
<font face="century gothic" size="3"> | <font face="century gothic" size="3"> | ||
| - | + | Water resources are priceless. Maintenance and reservation of clean natural water bodies are indispensable to us all. Our team, focusing on phosphate pollution, come up with the idea of E. capsi and try to improve the existing sewage treatment method by manipulating Synthetic Biology. | |
</font><br><br> | </font><br><br> | ||
<font face="impact" size="5" color="green"> | <font face="impact" size="5" color="green"> | ||
| Line 84: | Line 84: | ||
</font> | </font> | ||
<font face="century gothic" size="3"> | <font face="century gothic" size="3"> | ||
| - | + | Conventional method to remove phosphate is by chemical precipitation, yet the process is expensive and prone to have downstream handling problem. Learning from the Mother Nature, natural existing of phosphate-accumulating microorganisms (PAOs) attracted people attention and a promising Enhanced Biological Phosphorous Removal process is hence introduced for sewage treatment to remove excess phosphate. | |
<br> | <br> | ||
| - | + | Noticing the delicate relation between phosphate uptake and polyP synthesis, our team is trying to improve polyP synthesis efficiency by concentrating the polyP synthesis enzyme PPK1 into a protein cage called microcompartment. This microcompartment isolate the polyP synthesis reaction from reverse polyP degradation reaction by preventing the contact between polyP and polyP degradation enzymes. This idea may be useful in engineering exsiting PAOs as well as other organism candidate for better phosphate removal efficiency in sewage treatment. | |
| - | + | ||
| - | + | ||
<br><br><br> | <br><br><br> | ||
<font face="impact" size="5" color="green"> | <font face="impact" size="5" color="green"> | ||
| - | Utilizing Poly-P <br> Regeneration of ATP | + | Utilizing Poly-P <br><br> </font> |
| + | <font face="century gothic" size="3"> | ||
| + | Introduction of MCP not only help to improve polyP synthesis efficiency, it also isolates the polyP from other cytosolic matters. The MCPs just like small boxes packaged with polyP, it provides potentials that we can recover the aqueous environmental phosphate in the form of polyP. Embracing the 3R (Reduce, Reuse, Recycle) environmental sustaining principle, we find a way to reduce harmful phosphate from the environment, convert it into polyP and recover them for potential useful purpose.</font><br><br> | ||
| + | <font face="impact" size="5" color="green"> | ||
| + | Regeneration of ATP<br><br> | ||
</font> | </font> | ||
| Line 99: | Line 102: | ||
</font> | </font> | ||
<font face="impact" size="5" color="green"> | <font face="impact" size="5" color="green"> | ||
| - | Remediation of heavy metals | + | Remediation of heavy metals<br><br> |
</font> | </font> | ||
<font face="century gothic" size="3"> | <font face="century gothic" size="3"> | ||
| Line 107: | Line 110: | ||
</font> | </font> | ||
<font face="impact" size="5" color="green"> | <font face="impact" size="5" color="green"> | ||
| - | Antimicrobials | + | Antimicrobials<br><br> |
</font> | </font> | ||
<font face="century gothic" size="3"> | <font face="century gothic" size="3"> | ||
| Line 114: | Line 117: | ||
<br><br> | <br><br> | ||
<font face="impact" size="5" color="green"> | <font face="impact" size="5" color="green"> | ||
| - | It's all about E. Capsi | + | It's all about E. Capsi<br><br> |
</font> | </font> | ||
<font face="century gothic" size="3"> | <font face="century gothic" size="3"> | ||
Latest revision as of 03:54, 28 September 2013
Future Applications
Revisit to Our Targeted Problem
Water resources are priceless. Maintenance and reservation of clean natural water bodies are indispensable to us all. Our team, focusing on phosphate pollution, come up with the idea of E. capsi and try to improve the existing sewage treatment method by manipulating Synthetic Biology.
Creation, more than just Destruction
Conventional method to remove phosphate is by chemical precipitation, yet the process is expensive and prone to have downstream handling problem. Learning from the Mother Nature, natural existing of phosphate-accumulating microorganisms (PAOs) attracted people attention and a promising Enhanced Biological Phosphorous Removal process is hence introduced for sewage treatment to remove excess phosphate.
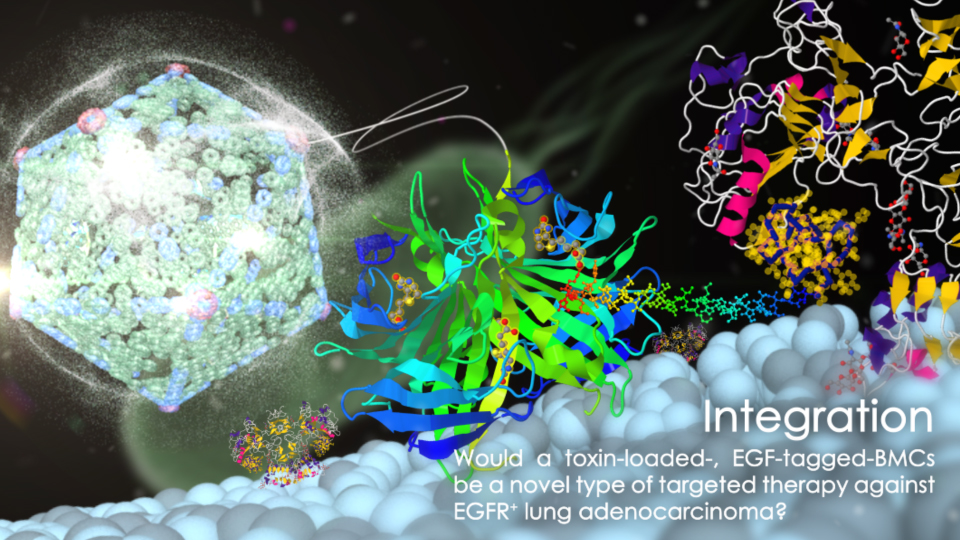
Noticing the delicate relation between phosphate uptake and polyP synthesis, our team is trying to improve polyP synthesis efficiency by concentrating the polyP synthesis enzyme PPK1 into a protein cage called microcompartment. This microcompartment isolate the polyP synthesis reaction from reverse polyP degradation reaction by preventing the contact between polyP and polyP degradation enzymes. This idea may be useful in engineering exsiting PAOs as well as other organism candidate for better phosphate removal efficiency in sewage treatment.
Utilizing Poly-P
Introduction of MCP not only help to improve polyP synthesis efficiency, it also isolates the polyP from other cytosolic matters. The MCPs just like small boxes packaged with polyP, it provides potentials that we can recover the aqueous environmental phosphate in the form of polyP. Embracing the 3R (Reduce, Reuse, Recycle) environmental sustaining principle, we find a way to reduce harmful phosphate from the environment, convert it into polyP and recover them for potential useful purpose.
Regeneration of ATP
As an enzymatic phosphorylating agent in industry, ATP is considerably expensive since its raw materials are highly costly reagents (i.e. acetyl phosphate, phosphoenolpyruvate, and creatine phosphate) in the enzymatic ATP-regenerating systems. Fortunately, poly P seems to be a perfectly suitable substitute of these reagents as ATP can be regenerated using immobilized PPK on a column. Using this method, each industrial form of poly P costing $0.25/lb can provide ATP that equivalents to what would have fetched over $2000. This greatly reduces the cost of ATP-related industries and provides new ways for many enzymatic reactions.
Remediation of heavy metals
Heavy metals such as mercury, copper and even radioactive uranium are devastating to the environment if they are left freely in the environment. Thanks to the advance in biotechnology, biomining of these harmful metals is made possible using poly P. Genetically engineered bacteria are utilized to take up and sequester the heavy metals and thus removing them from the environment. For example, the popular P. aeruginosa which can withstand lethal doses of irradiation removes uranyl ions in a poly P-facilitated reaction. Overexpression of PPK1 aids the removal of uranium by precipitation of uranyl phosphate.
Antimicrobials
Poly P has long been widely used as a food additive, especially in the meat and dairy industry to enhance flavor, water binding, color retention, and emulsification, while preventing oxidative rancidity, since there is virtually no cost and no health concerns regarding the use of poly P in food. Poly P also inhibits growth of bacteria, especially in high concentration. High concentrations of poly P are bacteriocidal which cause cell lysis. While with sublethal concentrations, it may affect septum formation that leads to the formation of multinucleate and filamentous cells. This inhibitory effects can be attributed to its ability to chelate cations. In addition, poly P also causes membrane damage in Staphylococcus aureus, a common infectious bacteria, by triggering the leakage of Mg2+ from cells, leading to an unbalanced osmotic pressure, and thus membrane damage. Naturally antibiotic-resistant gram-negative bacteria Stenotrophomonas maltophilia and Acinetobacter ssp. through the disk diffusion technique, have shown that membrane permeabilizers like poly P increased susceptibility to a wide variety of antibiotics, including imipenem, ciprofloxacin, tetracycline, and rifampicin. All in all, these effects are found to be due to the metal-chelating properties of poly P.
It's all about E. Capsi
Last but not least, it is obvious that the potential development in poly P-related industry is absolutely undeniable. In modern world of 21st century, the monotonic approach towards economic growth is no longer acceptable in any body's eyes. A "dual" approach which balances environmental conservation and economic growth is certainly a more desirable way to adopt. In fact, this is the main idea and our vision on E. capsi and we are not just aiming at a small change inside the microcompartment. We are aiming at a big change in the world!
Revisit to Our Targeted Problem
Water resources are priceless. Maintenance and reservation of clean natural water bodies are indispensable to us all. Our team, focusing on phosphate pollution, come up with the idea of E. capsi and try to improve the existing sewage treatment method by manipulating Synthetic Biology.
Creation, more than just Destruction
Conventional method to remove phosphate is by chemical precipitation, yet the process is expensive and prone to have downstream handling problem. Learning from the Mother Nature, natural existing of phosphate-accumulating microorganisms (PAOs) attracted people attention and a promising Enhanced Biological Phosphorous Removal process is hence introduced for sewage treatment to remove excess phosphate.
Noticing the delicate relation between phosphate uptake and polyP synthesis, our team is trying to improve polyP synthesis efficiency by concentrating the polyP synthesis enzyme PPK1 into a protein cage called microcompartment. This microcompartment isolate the polyP synthesis reaction from reverse polyP degradation reaction by preventing the contact between polyP and polyP degradation enzymes. This idea may be useful in engineering exsiting PAOs as well as other organism candidate for better phosphate removal efficiency in sewage treatment.
Utilizing Poly-P
Introduction of MCP not only help to improve polyP synthesis efficiency, it also isolates the polyP from other cytosolic matters. The MCPs just like small boxes packaged with polyP, it provides potentials that we can recover the aqueous environmental phosphate in the form of polyP. Embracing the 3R (Reduce, Reuse, Recycle) environmental sustaining principle, we find a way to reduce harmful phosphate from the environment, convert it into polyP and recover them for potential useful purpose.
Regeneration of ATP
As an enzymatic phosphorylating agent in industry, ATP is considerably expensive since its raw materials are highly costly reagents (i.e. acetyl phosphate, phosphoenolpyruvate, and creatine phosphate) in the enzymatic ATP-regenerating systems. Fortunately, poly P seems to be a perfectly suitable substitute of these reagents as ATP can be regenerated using immobilized PPK on a column. Using this method, each industrial form of poly P costing $0.25/lb can provide ATP that equivalents to what would have fetched over $2000. This greatly reduces the cost of ATP-related industries and provides new ways for many enzymatic reactions.
Remediation of heavy metals
Heavy metals such as mercury, copper and even radioactive uranium are devastating to the environment if they are left freely in the environment. Thanks to the advance in biotechnology, biomining of these harmful metals is made possible using poly P. Genetically engineered bacteria are utilized to take up and sequester the heavy metals and thus removing them from the environment. For example, the popular P. aeruginosa which can withstand lethal doses of irradiation removes uranyl ions in a poly P-facilitated reaction. Overexpression of PPK1 aids the removal of uranium by precipitation of uranyl phosphate.
Antimicrobials
Poly P has long been widely used as a food additive, especially in the meat and dairy industry to enhance flavor, water binding, color retention, and emulsification, while preventing oxidative rancidity, since there is virtually no cost and no health concerns regarding the use of poly P in food. Poly P also inhibits growth of bacteria, especially in high concentration. High concentrations of poly P are bacteriocidal which cause cell lysis. While with sublethal concentrations, it may affect septum formation that leads to the formation of multinucleate and filamentous cells. This inhibitory effects can be attributed to its ability to chelate cations. In addition, poly P also causes membrane damage in Staphylococcus aureus, a common infectious bacteria, by triggering the leakage of Mg2+ from cells, leading to an unbalanced osmotic pressure, and thus membrane damage. Naturally antibiotic-resistant gram-negative bacteria Stenotrophomonas maltophilia and Acinetobacter ssp. through the disk diffusion technique, have shown that membrane permeabilizers like poly P increased susceptibility to a wide variety of antibiotics, including imipenem, ciprofloxacin, tetracycline, and rifampicin. All in all, these effects are found to be due to the metal-chelating properties of poly P.
It's all about E. Capsi
Last but not least, it is obvious that the potential development in poly P-related industry is absolutely undeniable. In modern world of 21st century, the monotonic approach towards economic growth is no longer acceptable in any body's eyes. A "dual" approach which balances environmental conservation and economic growth is certainly a more desirable way to adopt. In fact, this is the main idea and our vision on E. capsi and we are not just aiming at a small change inside the microcompartment. We are aiming at a big change in the world!
 "
"