Team:NCTU Formosa/biobricks
From 2013.igem.org
(Difference between revisions)
Huierh04102 (Talk | contribs) (→BBa_K1017726) |
Huierh04102 (Talk | contribs) (→BBa_K1017781) |
||
| Line 16: | Line 16: | ||
======BBa_K1017781====== | ======BBa_K1017781====== | ||
| - | *This is the key part to change Ompc promoter intro red promotor. The lacI will inhibit the function of lac | + | *This is the key part to change Ompc promoter intro red promotor. The lacI will inhibit the function of P<sub>lac</sub>, thus, the original outcomes will be converted into our wanted result. |
| + | <br>[[File:Nctu_lacI_plac.jpg|200px]] | ||
======BBa_K1017101====== | ======BBa_K1017101====== | ||
Revision as of 10:35, 20 October 2013
Biobricks
To strive for the best results, we made a number of experiment. Our team always hang together in spite of many losses.
Contents |
Parts submitted to the Registry
Light-regulated system
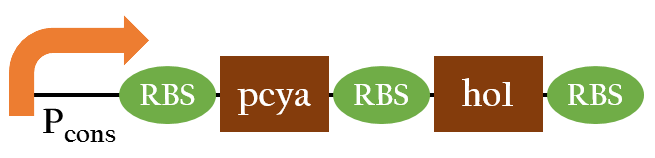
BBa_K1017726
- pcya and ho1 are the enzymes needed to convert heme into chromophore phycocyanobiline(PCB), the red light sensor. Since pcya and ho1 are not naturally produced in E.coli, we use Pcons upstream in order to make E.coli continuously expressed them to synthesize PCB.
BBa_K1017301
- Cph8 is a chimeric light receptor. It is a fusion of the photoreceptor Cph1 and the EnvZ histidine kinase. Cph1 is only active when it binds the chromophore phycocyanobiline (PCB).
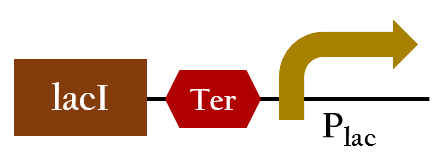
BBa_K1017781
- This is the key part to change Ompc promoter intro red promotor. The lacI will inhibit the function of Plac, thus, the original outcomes will be converted into our wanted result.
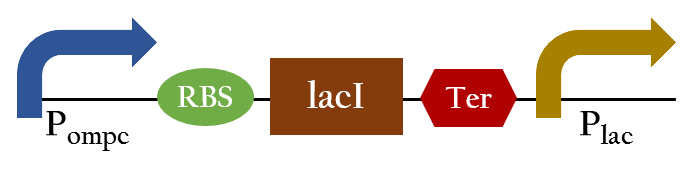
BBa_K1017101
- Ompc promtor, which can sense red light with the presence of cph8. It will be turned on in the dark ,and be turned off in the bright. For the convenient use, we add lacI and lac promotor downstream the biobrick. This part, so called red promotor, can be activated under red light, and inactive in the dark.
Small RNA-regulated system
BBa_K1017403
BBa_K1017404
BBa_K1017202
- The sRNA, base pair with target mRNA, including the Shine-Dalgarno sequence. Thus it prevent ribosome from binding to initiate the translation. The rRBS is designed for sRNA perfect binding, and this rRBS is the RBS which can be bound only for our artificial sRNA(BBa_K1017404).
BBa_K1017811
BBa_K1017401
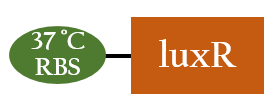
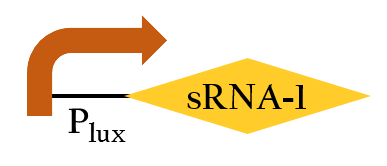
- This part, BBa_K1017401, includes our artificial sRNA-1 and rRBS-1. The non-coding small RNA can bind to the Shine-Dalgarno sequence on rRBS-1 by base-pairing. Once the rRBS-1 is blocked, ribosomes cannot bind to it to translate, thus, gene expressions downstream are decreased. Because of specific binding, rRBS-1 can only be bound by sRNA-1. We add Plux upstream, so this part can be regulated by luxR/AHL complex.
BBa_K1017402
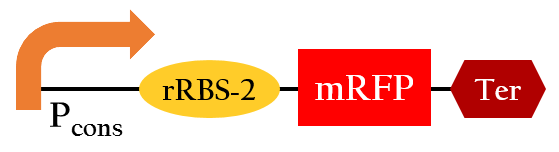
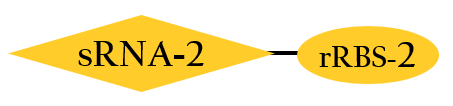
- This part, BBa_K1017402, is similar to BBa_K1017401 mentioned above, but without Plux. rRBS-2 can only be bound by sRNA-2 due to specificity, then gene expressions downstream are decreased.
<groupparts>iGEM013 NCTU_Formosa</groupparts>
 "
"