From 2013.igem.org
22-07-2013
Re-PCR from FS_10 to FS_23; 3.3 kb; 13-07-2013

Re-PCR of DelG (10-23), Amplification of DelG (8-23) (22.07); run at 100 V, 0.8 % gel (TAE)

Re-PCR of DelG (10-23), Amplification of DelG (8-23) (22.07) cut; run at 100 V, 0.8 % gel (TAE)
- Reaction
| what | µl
|
| Fragment FS_10 to FS_11_short (13-07-2013) | 1
|
| FS_10: (1/10) | 2
|
| FS_23: (1/10) | 2
|
| Phusion flash Master Mix | 10
|
| DMSO | 1
|
| dd H2O | 4
|
- Conditions
--> First cycles with wrong program
| Biorad C1000 Touch Block A
|
| Cycles | temperature [°C] | Time [s]
|
| 1 | 98 | 10
|
| 12 | 98 | 1
|
| 68 ↓ 0.5 | 5
|
| 72 | 1:10 min
|
| 18 | 98 | 1
|
| 66 | 5
|
| 72 | 1:10 min
|
| 1 | 72 | 5 min
|
| 1 | 12 | inf
|
Results:
- Re-Amplification of DelG lead to the expected fragment but also to a smear, therefore fragment will not be used for Gibson Assembly but restriction digests to validate the product.
Amplification from FS_08 to FS_23; 6.5 kb
- Reaction
| what | µl
|
| D. acidovorans DSM-39 | 1
|
| FS_08: (1/10) | 2
|
| FS_23: (1/10) | 2
|
| Phusion flash Master Mix | 10
|
| DMSO | 1/-
|
| dd H2O | 4/-
|
- Conditions
| Biorad MyCycler*
|
| Cycles | temperature [°C] | Time [s]
|
| 1 | 98 | 10
|
| 12 | 98 | 1
|
| 66 ↓ 0.5 | 5
|
| 72 | 3:20
|
| 18 | 98 | 1
|
| 64 | 5
|
| 72 | 3:20
|
| 1 | 72 | 10min
|
| 1 | 12 | inf
|
Results:
- Amplification of DelG worked, leading to the expected product as well one other unexpected band.
- bands were cut out and DNA purified using QIAquick Gel Extraction Kit
Amplification from FS_08 to FS_11s; 6.5 kb
- Reaction
| what | µl
|
| D. acidovorans DSM-39 | 1
|
| FS_08: (1/10) | 2
|
| FS_11_short: (1/10) | 2
|
| Phusion flash Master Mix | 10
|
| DMSO | 1
|
| dd H2O | 4
|
- Conditions
| Biometra TProfessional Basic
|
| Cycles | temperature [°C] | Time [s]
|
| 1 | 98 | 10
|
| 14 | 98 | 1
|
| 62 ↓ 0.5 | 5
|
| 72 | 3:20
|
| 16 | 98 | 1
|
| 60 | 5
|
| 72 | 3:20
|
| 1 | 72 | 10min
|
| 1 | 12 | inf
|
23-07-2013
Amplification from FS_08 to FS_23; 6.5 kb
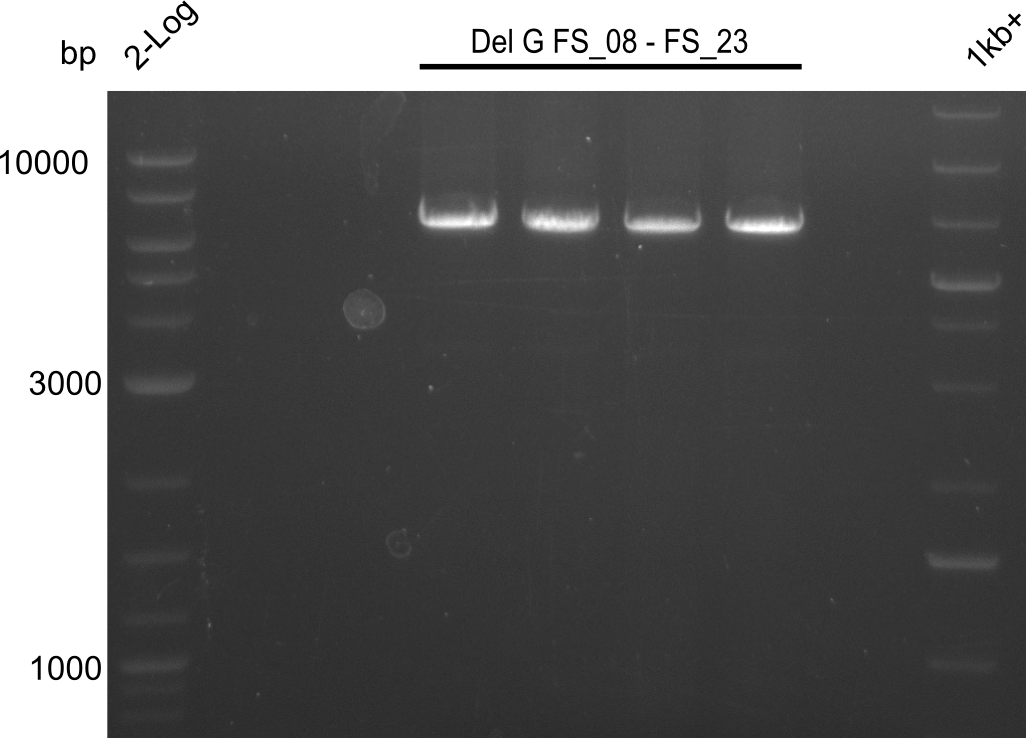
Amplification of DelG (FS_08-FS_23); run at 100 V, 0.8 % gel (TAE)
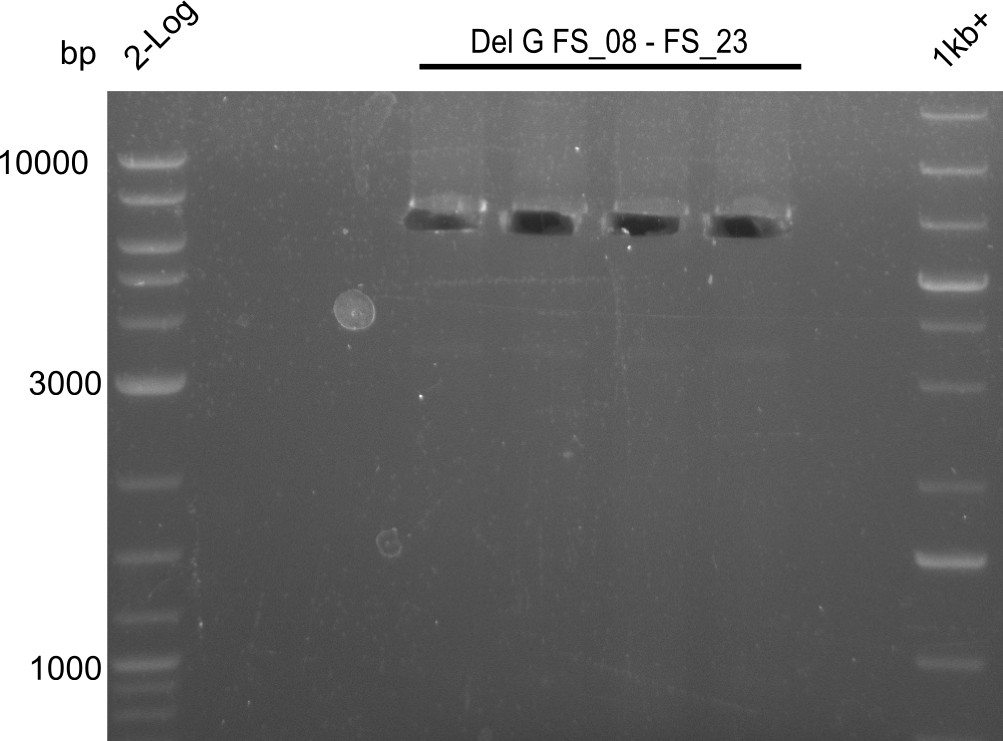
Amplification of DelG (FS_08-FS_23)after cutting; run at 100 V, 0.8 % gel (TAE)

Measurement of concentration of DelG, DelFG and DelOP (2µl of each); run at 100 V, 0.8 % gel (TAE)
4x 20µl
- Reaction
| what | µl
|
| D. acidovorans DSM-39 | 1
|
| FS_08: (1/10) | 2
|
| FS_23: (1/10) | 2
|
| Phusion flash Master Mix | 10
|
| DMSO | 1
|
| dd H2O | 4
|
- Conditions
| Biometra TProfessional Basic
|
| Cycles | temperature [°C] | Time [s]
|
| 1 | 98 | 10
|
| 12 | 98 | 1
|
| 66 ↓ 0.5 | 5
|
| 72 | 3:20
|
| 18 | 98 | 1
|
| 64 | 5
|
| 72 | 3:20
|
| 1 | 72 | 10min
|
| 1 | 12 | inf
|
Results:
- Amplification of DelG was successful
- bands were cut out and DNA purified using QIAquick Gel Extraction Kit
- Gel to proof quality of gel extraction was run
26-07-2013
Restriction digest of Fragment FS_08 to FS_23; 6.5 kb; 23-07-2013 with BglII
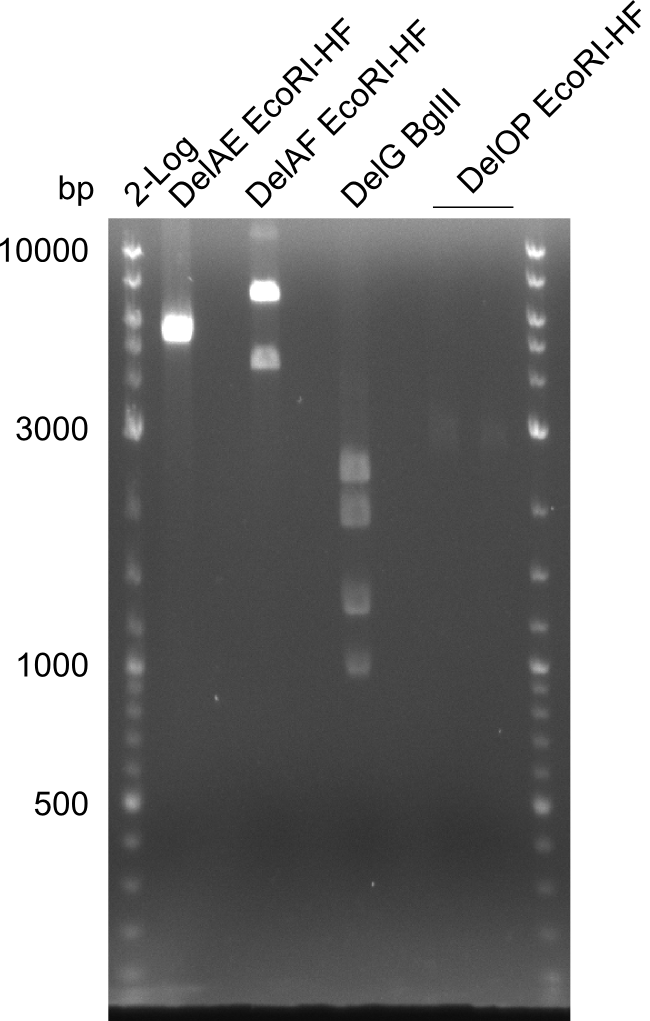
Restriction digest of DelAE (FS_02 to FS_03 ;08.07), DelAF (FS_02 to FS_05; 04.07) and DelOP (FS_22 to FS_13short ;21.07) with EcoRI-HF and digest of DelG (FS_08 to FS_23 cut1 ;23.07) with BglII; run at 100 V, 0.8 % gel (TAE)
Incubation at 37°C for 1hour 45min
| what | µl
|
| FS_08 to FS_23 cut1 (23-07-2013) | 15
|
| BglII | 0.5
|
| Buffer 3.1 | 2
|
| dd H2O | 2
|
| Expected fragment lengths [bp] | 3291, 1936, 1303
|
Results:
- Restriction digest of DelG (FS_08 to FS_23) was successful though one unexpected band (presumably carry over) appeared
- Construct will be further validated by sequencing before potential Gibson Assembly
 "
"





