From 2013.igem.org
12-08-2013
Amplification I from FS_22 to FS_13; 2.7 kb
- Reaction
| Reagent | DelOP
|
| Template | D.acidovorans SPH-1 colony | 1µl FS_22-FS_13_short 10-08
|
| Primer fw | 2 µl FS_22
|
| Primer rev | 2 µl FS_13
|
| Phusion Ready Mix | 10 µl
|
| dd H2O | 5 µl
|
- Conditions
| Biorad MyCycler*
|
| Cycles | temperature [°C] | Time [s]
|
| 1 | 98 | 10
|
| 30 | 98 | 1
|
| 55 | 5
|
| 72 | 1:00
|
| 1 | 72 | 5min
|
| 1 | 10 | inf
|
Results:
- Amplification of DelOP did not work
- PCR will be repeated at very high annealing temperatures to ensure that primers with very complex secondary structure such as the Gibson-Primer FS_13long are able to bind
Amplification II and III from FS_22 to FS_13; 2.7 kb
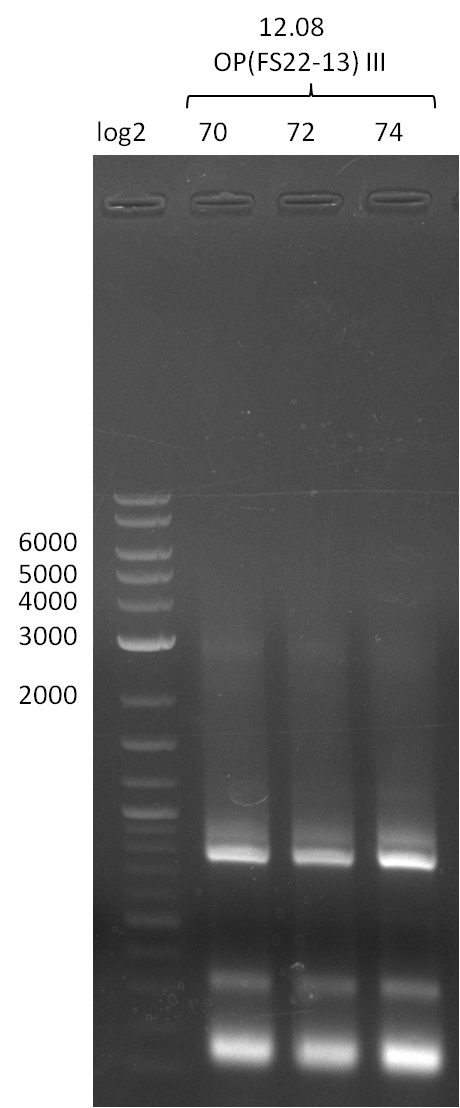
Amplification of DelOP III .; run at 100 V, 0.8 % gel (TAE)(12.08)
Amplification of DelOP 72°C const.; run at 135 V, 0.8 % gel (TAE)(11.08)
Amplification of DelOP 72°C const. after cutting; run at 135 V, 0.8 % gel (TAE)(11.08)
- Reaction
| Reagent | DelOP
|
| Template | 1µl FS_22-FS_13_short 10.08
|
| Primer fw | 4 µl FS_22
|
| Primer rev | 4 µl FS_13
|
| Phusion Ready Mix | 10 µl
|
| DMSO | 1 µl
|
- Conditions
| Biorad MyCycler
|
| Cycles | temperature [°C] DelOP II | Time | | Cycles | temperature [°C] DelOP III | Time
|
| 1 | 98 | 10 s | | 1 | 98 | 10 s
|
| 30 | 98 | 1 s | 12 | 98 | 1 s
|
| 74.0 - 70.0 (ΔT = 2.0) ↓ 0.5 | 5 s
|
| 72 | 1:00 min | 72 | 3:15 min
|
| 18 | 98 | 1 s
|
| 66 | 5 s
|
| 68 / 70 / 72 | 1:00 min
|
| 1 | 72 | 10 min | 1 | 72 | 10 min
|
| 1 | 10 | inf | 1 | 10 | inf
|
Results:
- Amplification of DelOP was successful, 2-step PCR with FS_22-FS_13s as template led to expected bands as well as a slight smear and an unintended product
- bands were cut out and DNA purified using QIAquick Gel Extraction Kit
- PCR will be repeated to obtain more sample
Re-PCR from FS_22 to FS_13; 2.7 kb; 10-08-2013)
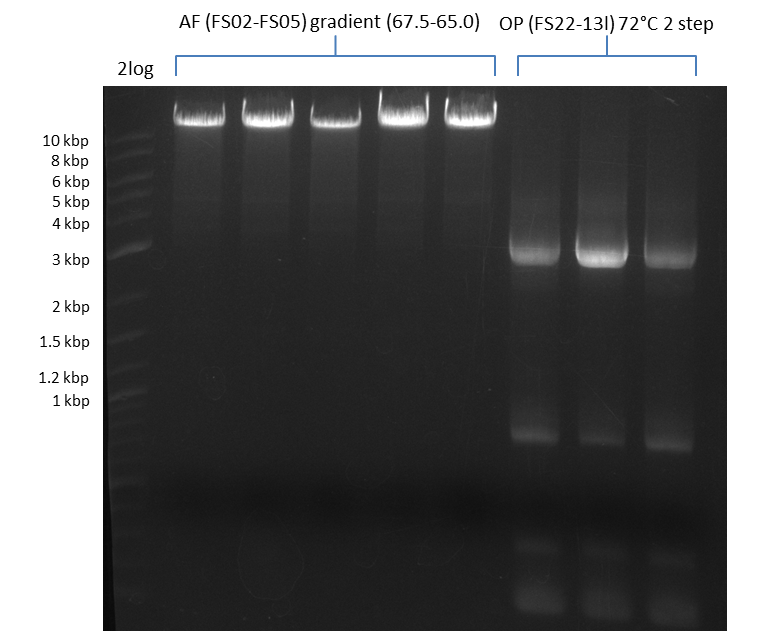
Amplification of DelAF using gradient PCR, Amplification of DelOP 72°C 2-step; run at 135 V, 0.8 % gel (TAE)(11.08)
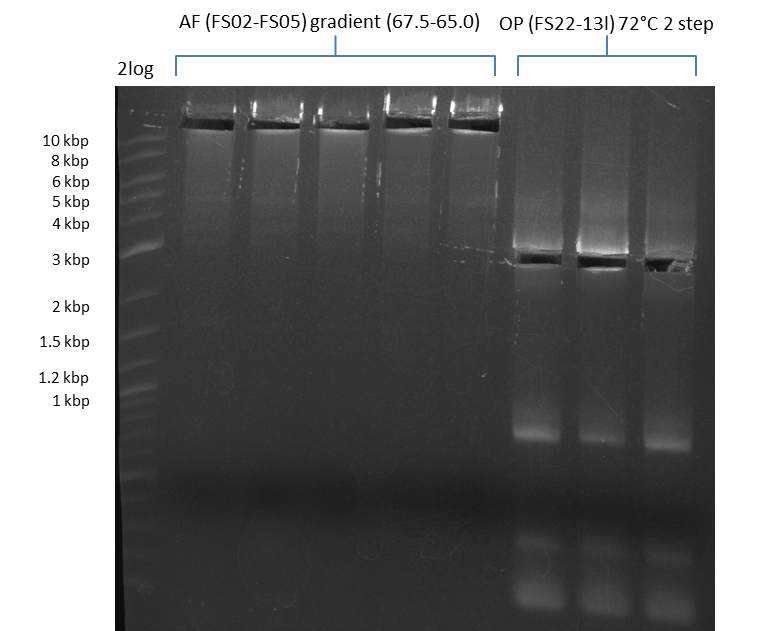
Amplification of DelAF using gradient PCR, Amplification of DelOP 72°C 2-step after cutting; run at 135 V, 0.8 % gel (TAE)(11.08)
3x 20µl
- Reaction
| Reagent | DelOP
|
| Template | 1µl FS_22-FS_13_short 10-08-2013
|
| Primer fw | 4 µl FS_22
|
| Primer rev | 4 µl FS_13
|
| Phusion Ready Mix | 10 µl
|
| DMSO | 1 µl
|
- Conditions
| Biorad T100
|
| Cycles | temperature [°C] | Time [s]
|
| 1 | 98 | 10
|
| 30 | 98 | 1
|
| 72 | 1:05
|
| 1 | 72 | 5 min
|
| 1 | 10 | inf
|
Results:
- Amplification of DelOP was successfull
- bands were cut out and DNA purified using QIAquick Gel Extraction Kit
- gradient PCR with annealing at 74°C, 72°C and 70°C will be carried out to increase yield
Amplification V from FS_22 to FS_13; 2.7 kb
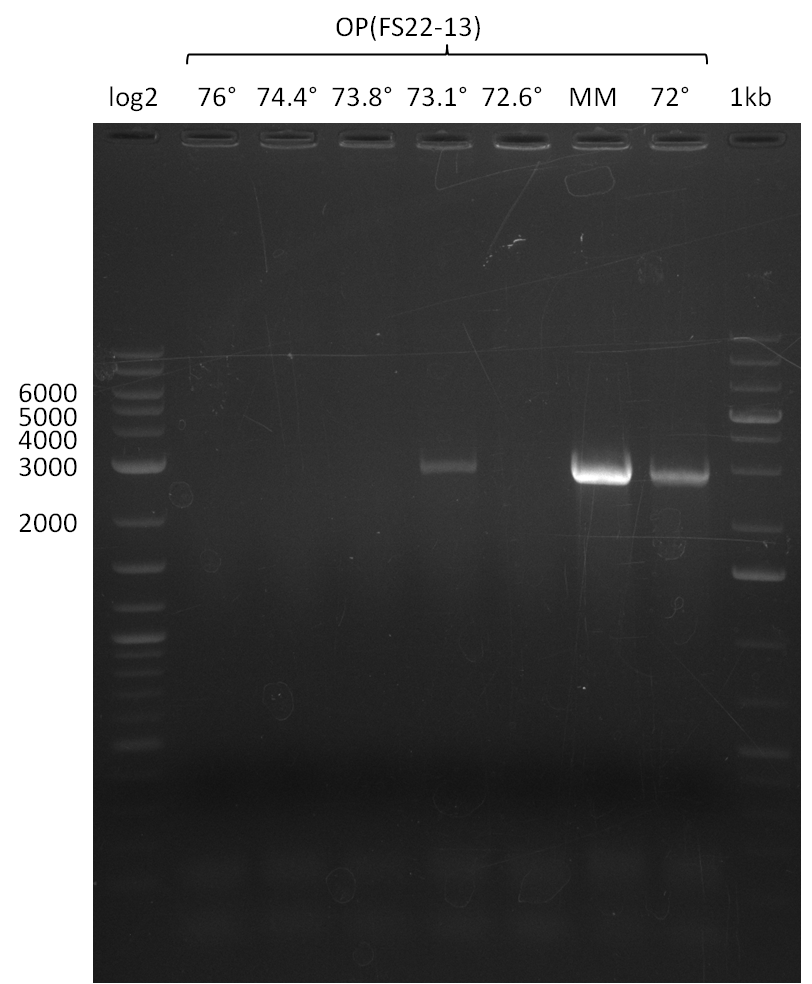
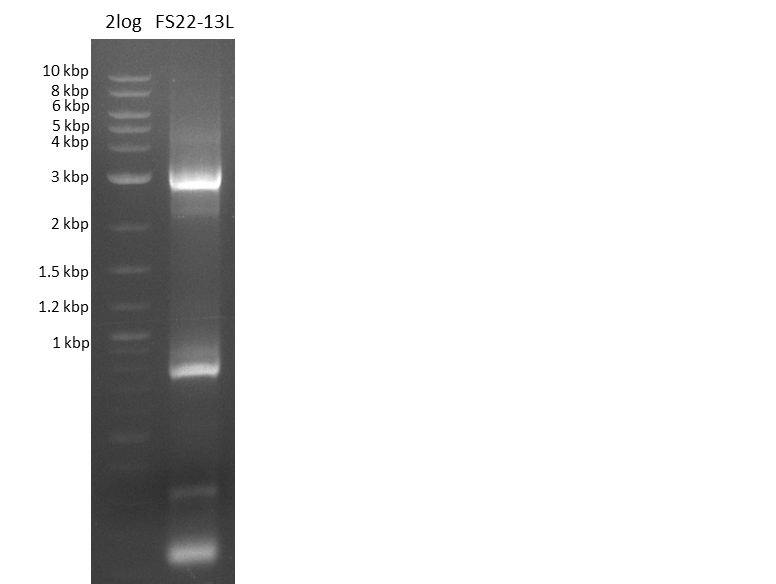
Amplification of DelOP V (12-08-13); run at 100 V, 0.8 % gel (TAE)

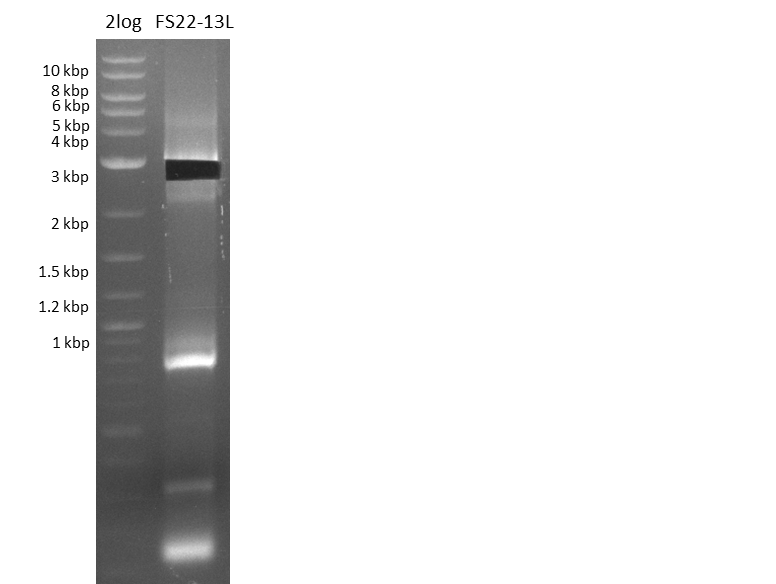
Amplification of DelOP V (12-08-13) cut
- Reaction
| Reagent | DelOP
|
| Template | D.acidovorans SPH-1 colony
|
| Primer fw | 4.5 µl FS_22
|
| Primer rev | 4.5 µl FS_13
|
| Phusion Ready Mix | 10 µl
|
| DMSO | 1 µl
|
- Conditions
| Biorad T100
|
| Cycles | temperature [°C] | Time [s]
|
| 1 | 98 | 10
|
| 30 | 98 | 1
|
| 75.0 - 72.0 (ΔT = 0.5) | 1:05
|
| 1 | 72 | 5 min
|
| 1 | 10 | inf
|
Results:
- Amplification of DelOP was successfull
- The gradient PCR shows that amplification worked best with an annealing temperature of 72.3°C, interestingly this annealing temperature was used for the remaining mastermix of the gradient PCR which included more DMSO than the other samples as mixing of DMSO often is not perfect and therefore the very last sample is of higher DMSO concentration
- Therefore PCR will be repeated with an annealing temperature of 72.3°C and 10% DMSO
13-08-2013
Restriction digest of fragment FS_22 to FS_13; 2.7 kb; 12-08-2013) with EcoRI-HF
Incubation at 37°C for 2 hours
| what | µl
|
| FS_22 to FS_13 (12-08-2013) | 20
|
| EcoRI-HF | 1
|
| CutSmart Buffer | 2.5
|
| dd H2O | 1.5
|
| Expected fragment lengths [bp] | 1883, 960
|
Results:
- restriction digest shows the expected bands, therefore validation if PCR amplicon is sufficient for Gibson Assembly
Amplification from FS_22 to FS_13; 2.7 kb
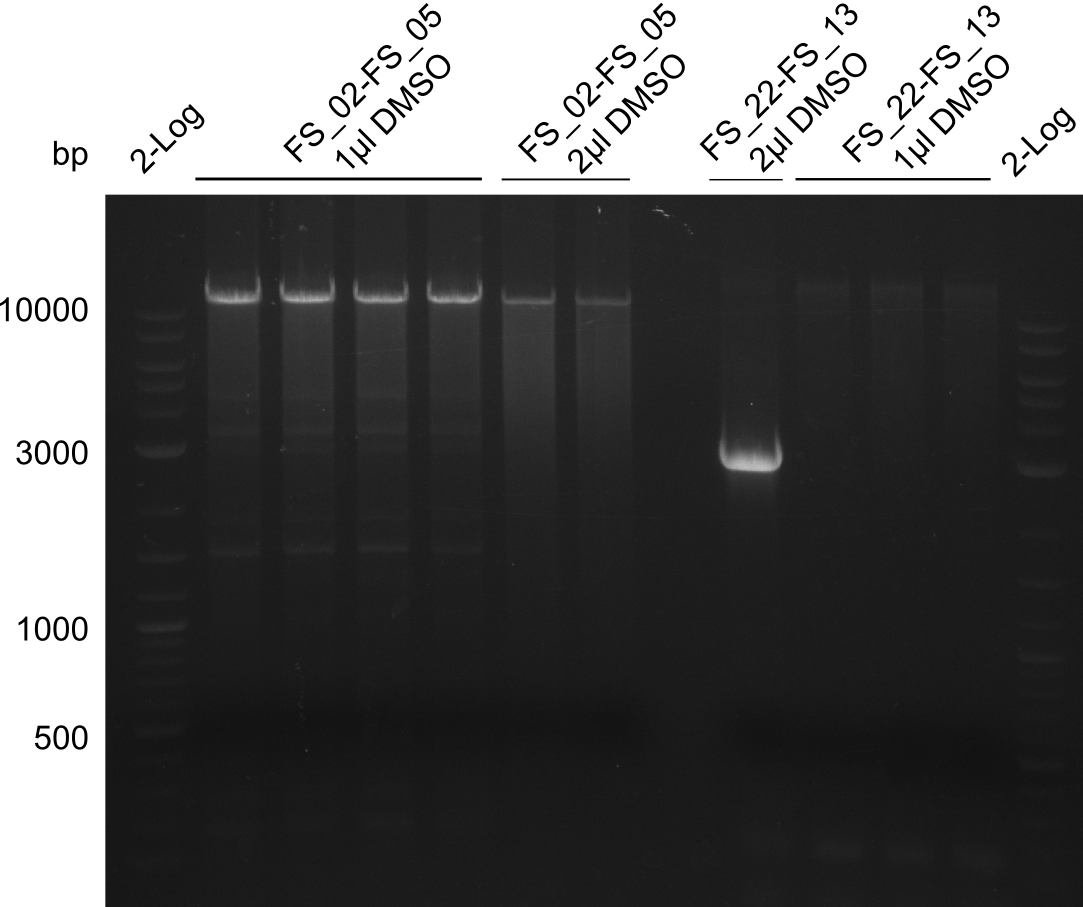
Amplification of DelAF (FS_02-FS_05); run at 100 V, 0.8 % gel (TAE)(13.08)
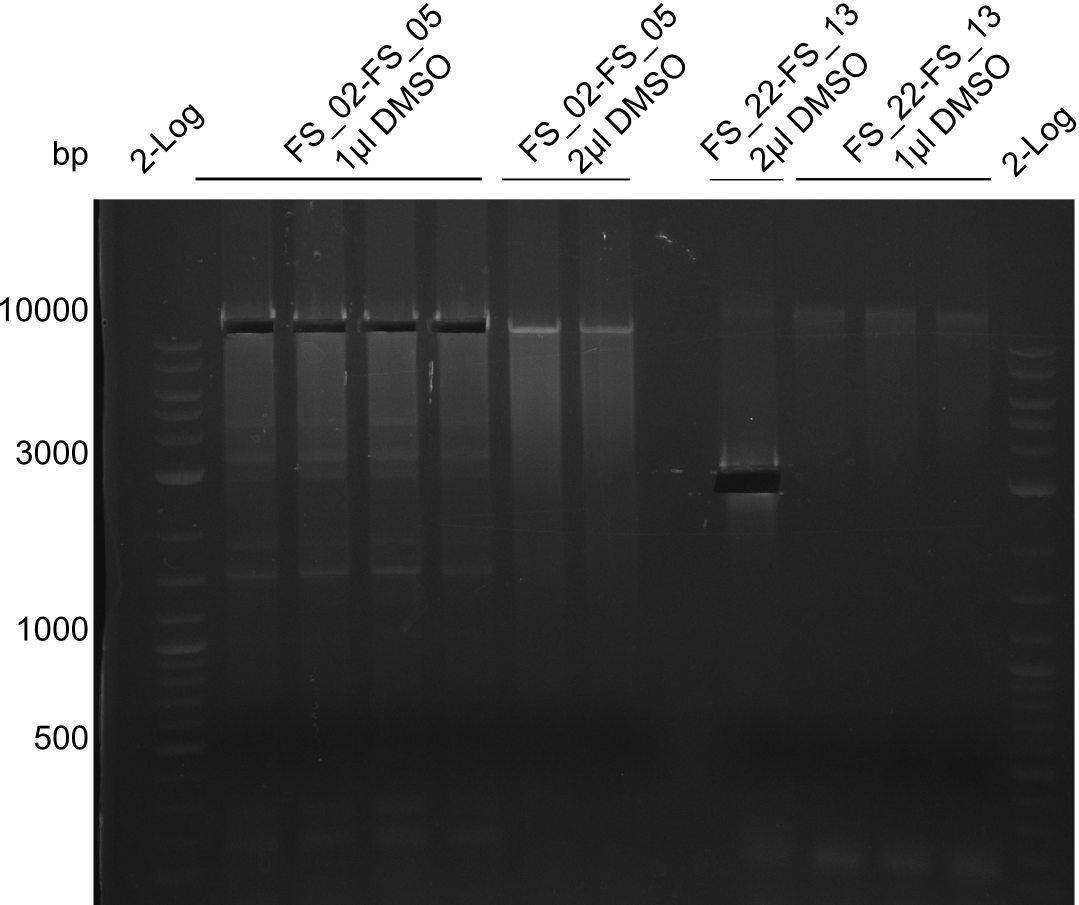
Amplification of DelAF (FS_02-FS_05), cut; run at 100 V, 0.8 % gel (TAE)(13.08)
- Reaction
| Reagent | DelOP
|
| Template | D.acidovorans SPH-1 colony
|
| Primer fw | 4 µl FS_22
|
| Primer rev | 4 µl FS_13
|
| Phusion Ready Mix | 10 µl
|
| DMSO | 1/2 µl
|
| dd H2O | 1/- µl
|
- Conditions
| Biorad T100
|
| Cycles | temperature [°C] | Time [s]
|
| 1 | 98 | 10
|
| 30 | 98 | 1
|
| 72 | 1:05
|
| 1 | 72 | 5 min
|
| 1 | 10 | inf
|
Results:
- Amplification of DelOP was successfull with 10% DMSO leading to one specific band of intended size
- PCR will be repeated with 10% DMSO to obtain the amount of product which is necessary for Gibson Assembly and test restriction digest
14-08-2013
Concentration measurement DelOP
| Fragment | Primer | Date PCR | Concentration
|
| DelOP | FS22-FS13 | 12-08-2013 | 11 ng/µl
|
17-08-2013
Amplification from FS_22 to FS_13; 2.7 kb
6x20 µl
- Reaction
| Reagent | DelOP
|
| Template | D.acidovorans SPH-1 colony
|
| Primer fw | 2.5 µl FS_22
|
| Primer rev | 2.5 µl FS_13
|
| Phusion Ready Mix | 10 µl
|
| DMSO | 2 µl
|
| dd H2O | 3 µl
|
- Conditions
| Biorad T100
|
| Cycles | temperature [°C] | Time [s]
|
| 1 | 98 | 10
|
| 30 | 98 | 1
|
| 72.3 | 5
|
| 72 | 1:00
|
| 1 | 72 | 5 min
|
| 1 | 10 | inf
|
18-08-2013
Amplification from FS_22 to FS_13; 2.7 kb
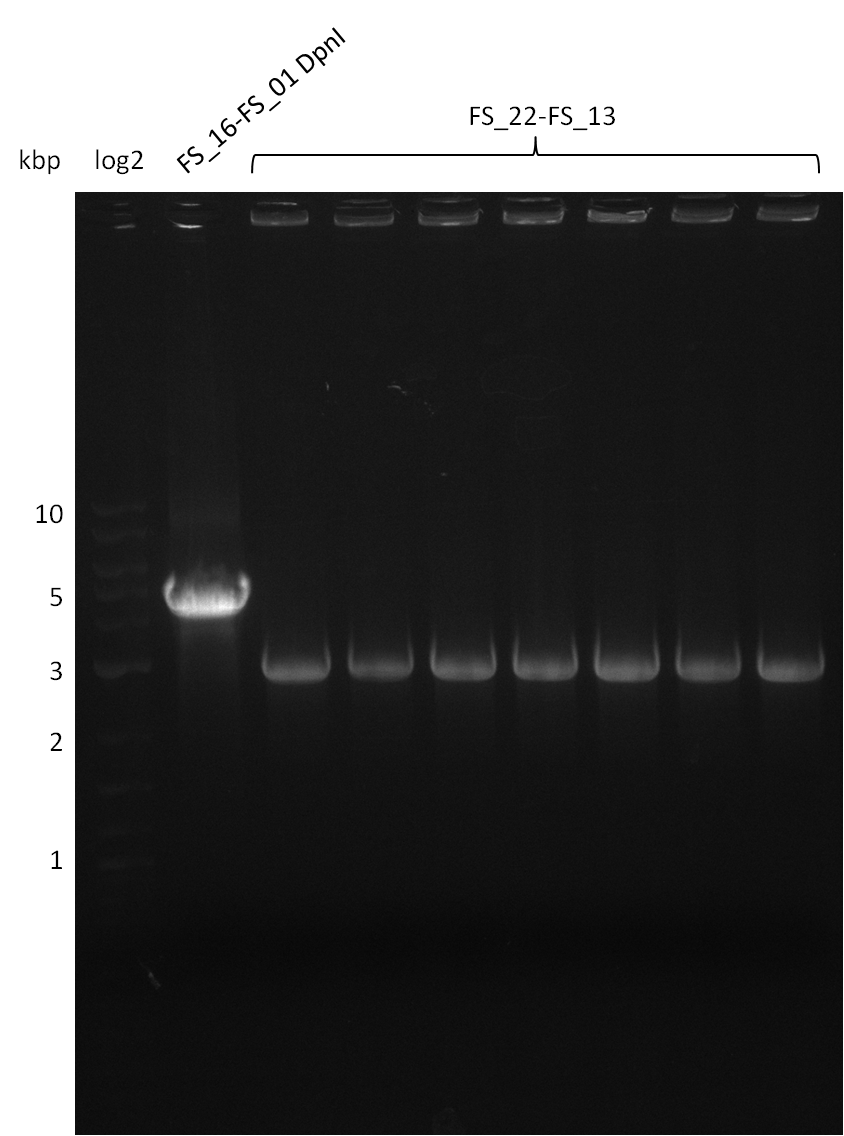
Restriction digest of pSB4K5 (17-08) with DpnI (17-08) and amplification of DelOP (18-08); run at 100 V, 0.8 % gel (TAE)
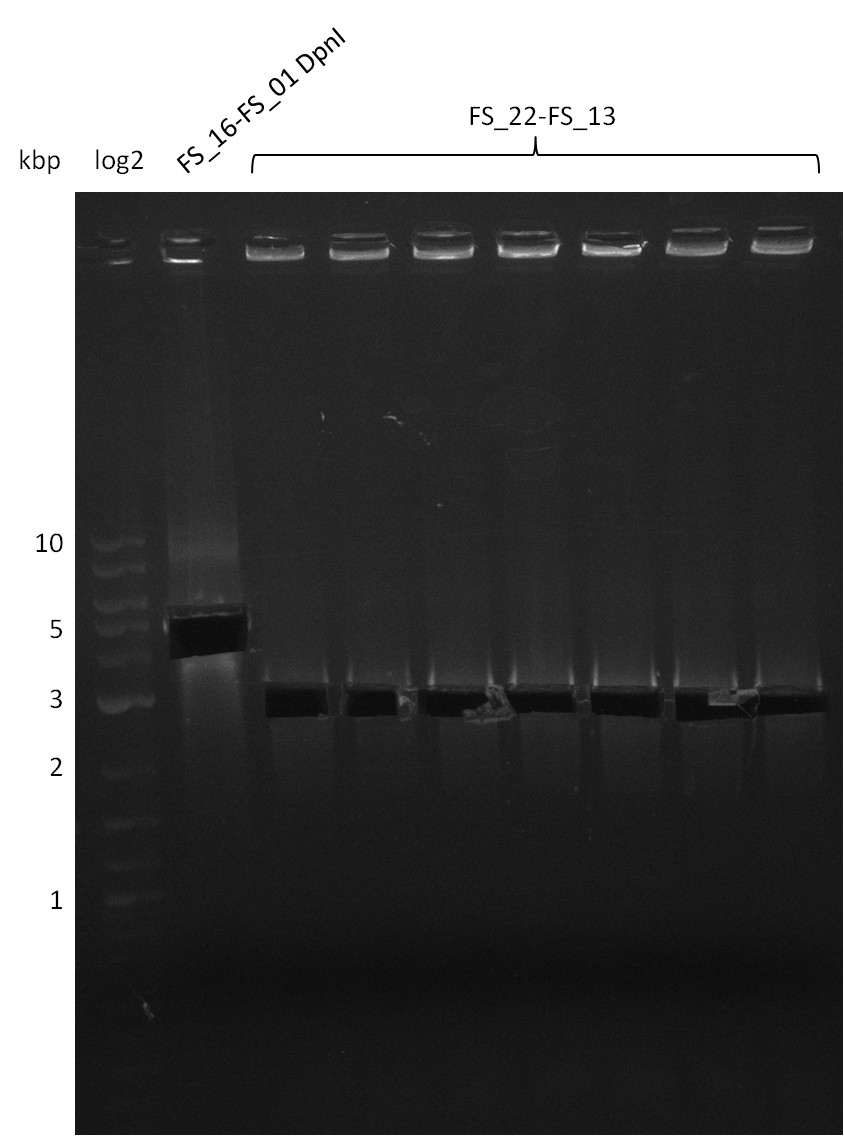
Restriction digest of pSB4K5 (17-08) with DpnI (17-08) and amplification of DelOP, cut (18-08); run at 100 V, 0.8 % gel (TAE)
6x20 µl
- Reaction
| Reagent | DelOP
|
| Template | D.acidovorans SPH-1 colony
|
| Primer fw | 2.5 µl FS_22
|
| Primer rev | 2.5 µl FS_13
|
| Phusion Ready Mix | 10 µl
|
| DMSO | 2 µl
|
| dd H2O | 3 µl
|
- Conditions
| Biorad T100
|
| Cycles | temperature [°C] | Time [s]
|
| 1 | 98 | 10
|
| 30 | 98 | 1
|
| 72.3 | 5
|
| 72 | 1:00
|
| 1 | 72 | 5 min
|
| 1 | 10 | inf
|
Results:
- Amplification of DelOP was successful
- bands were cut out and DNA purified using QIAquick Gel Extraction Kit
 "
"










