Team:Heidelberg/Templates/Delftibactin week3
From 2013.igem.org
Contents |
13-05 - 19-05-13
Gold Recovery from Electronic Waste
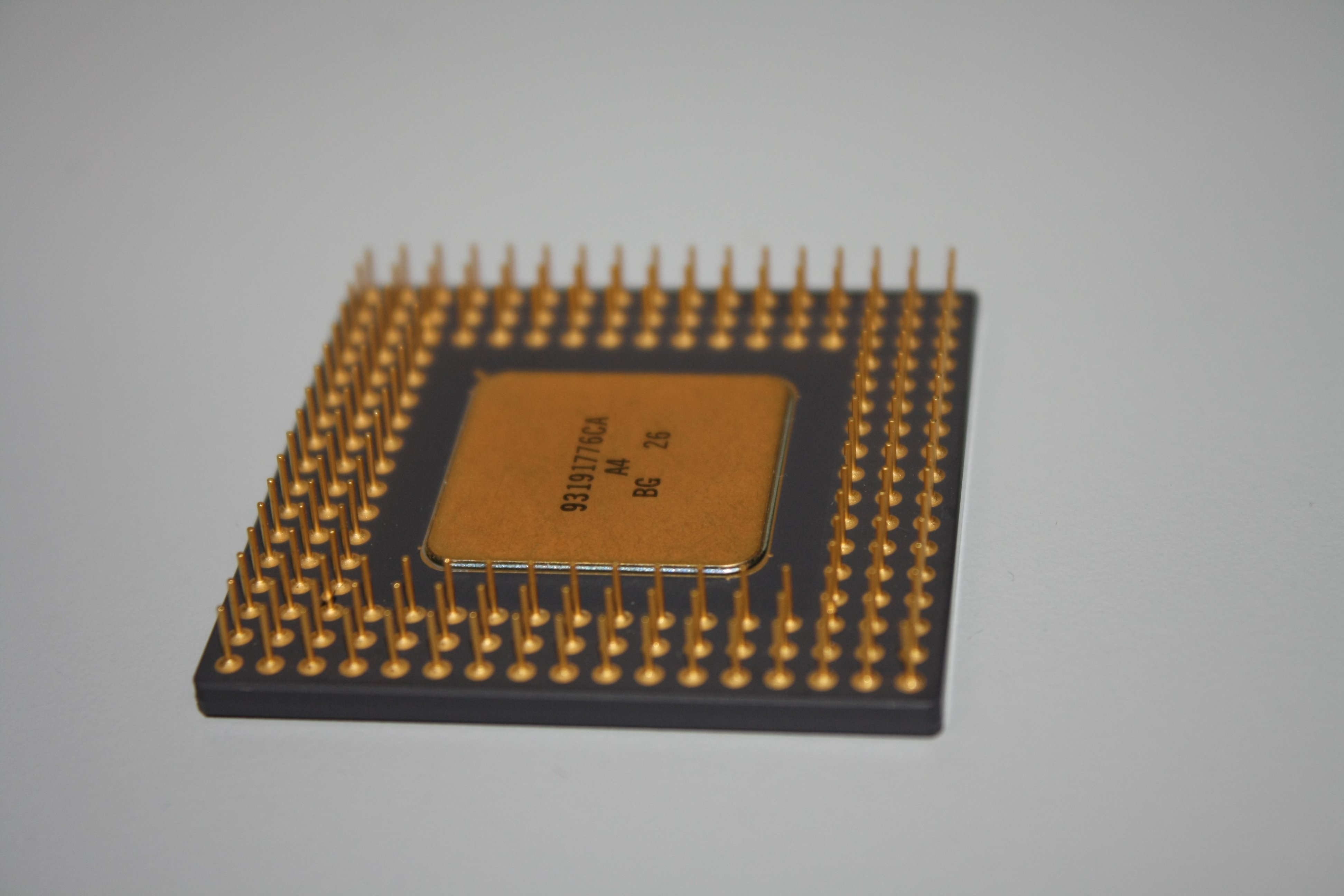
As test experiment, pins from an old CPU processor chip were dissolved.
Step 1: Pins were mechanically removed and collected in a flask.
Step 2: Flask was put onto heating plate and covered in concentrated HCl.
Step 3: HNO3 was carefully added to the pins and heated.
Step 4: After several minutes of moderate heating, pins dissolved completely. The solution turned green.
Step 5: The solution was carefully neutralized to pH ~6 in a step-wise manner using first 5 M, later 0.5 M NaOH.
Step 6: This led to precipitation of copper salts, indicated by the milky green coloring.
Step 7: In order to remove the precipitates, the solution was filtered using a buechner funnel and sterile filtrated (0.2 µm).
Step 8: The final yellow-green gold containing solution was collected in a volumetric flask.
Precipitation of Gold from Electronic Waste
Protocol
In order to evaluate optimial cultivation temperature of D. acidovorans, glycerol stock was streaked on ACM plates and incubated at RT, 30°C and 37°C ON. The next day, plates were covered with 200 µl 0.5% soft agar supplemented with 27 µl "dissolved electronic waste" solution.
Result
D. acidovorans showed intermediate, postponed purple-black coloring upon addition of "dissolved electronic waste" soft agar. Strongest coloring was observed on plates incubated at room temperature and 30°C. Delftibactin expression seems to be significantly decreased in D. acidovorans cultivated at 37°C. Reduced coloring further indicates lower gold ion concentration in the solution recovered compared to th ecommercially availible gold chloride solution [100 g/l]
We could therefore prove capability of D. acidovorans to specifically recover solid gold from dissolved electronic waste.
 "
"