Team:Heidelberg/Templates/MM week20p
From 2013.igem.org
Contents |
2013-09-09
- co-transform TOP10 with pRB22.3 and pIK7.1, plate on Cm+Kan, grow at 37°C
2013-09-10
- after ca. 18h at 37°C: lots of tiny colonies, not blue
- leave at RT in drawer (no light)
2013-09-11
- colonies became blue
- start constructing pIK8: promoter even weaker than the one used by Cambridge 2007, weak RBS
- run PCRs of (2 ng DNA, 20 µl total volume, using Q5):
- pIK2.6 with primers IK40+IK43 -> f7
| Cycles | temperature [°C] | Time [s] |
|---|---|---|
| 1 | 98 | 30 |
| 35 | 98 | 10 |
| 58 | 10 | |
| 72 | 300 | |
| 1 | 72 | 600 |
| 1 | 10 | inf |
- BBa_I746200 with primers IK41+IK42 -> f8
| Cycles | temperature [°C] | Time [s] |
|---|---|---|
| 1 | 98 | 30 |
| 35 | 98 | 10 |
| 60 | 10 | |
| 72 | 90 | |
| 1 | 72 | 600 |
| 1 | 10 | inf |
2013-09-12
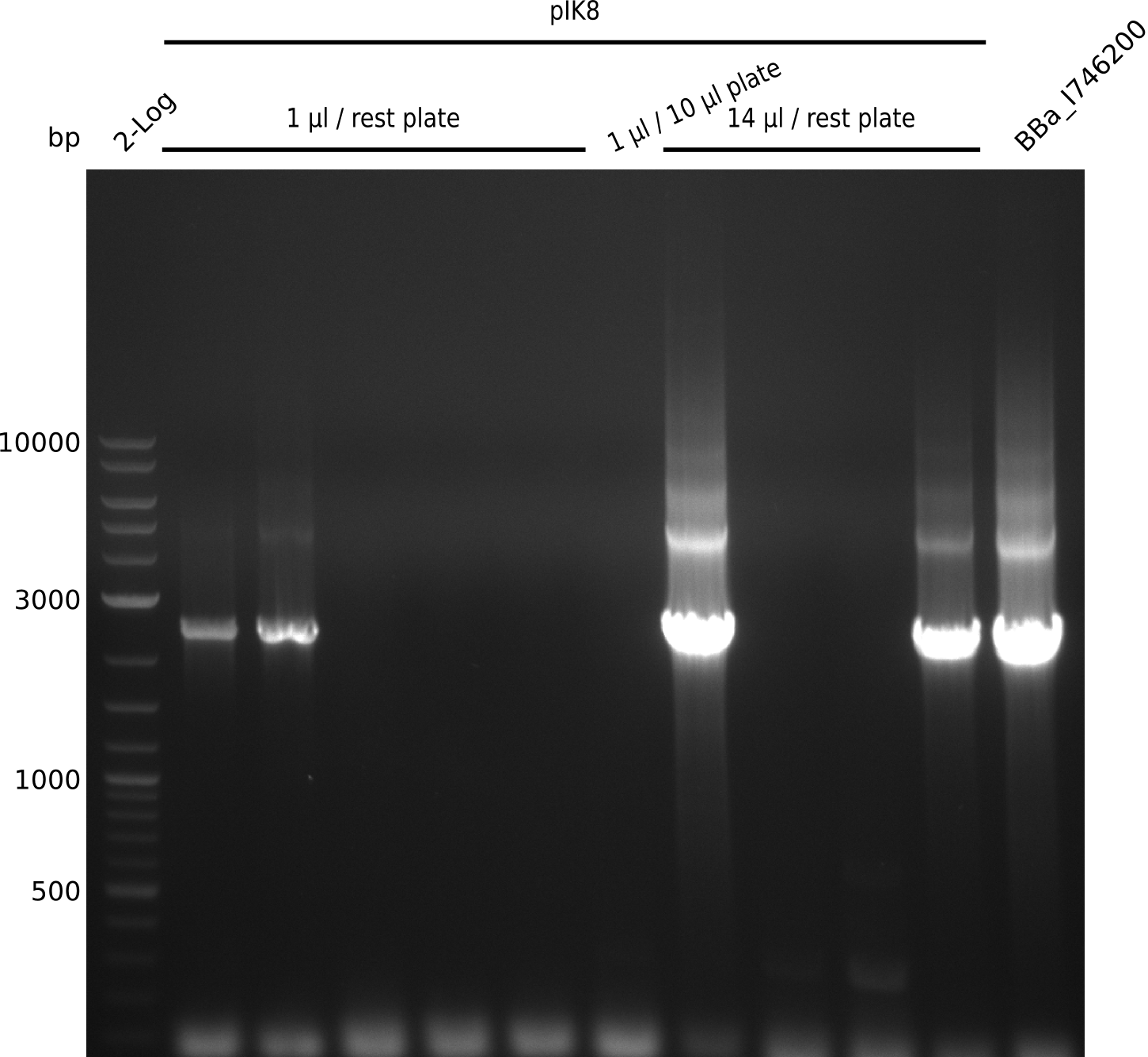
- run gel, gel-purify fragments -> 6.3 ng/µl in 20 µl (f7); 36.1 ng/µl in 20 µl (f8)
- perform Gibson assembly: 8 µl f7, 2 µl f8, 10 µl MM, incubate at 50°C for 1h
- dilute Gibson mix 1:4, electroporate electrocompetent DH10ß with 1 µl, 14 µl of dilution
- plate 10 µl, rest on Cm, grow at 37°C
2013-09-13
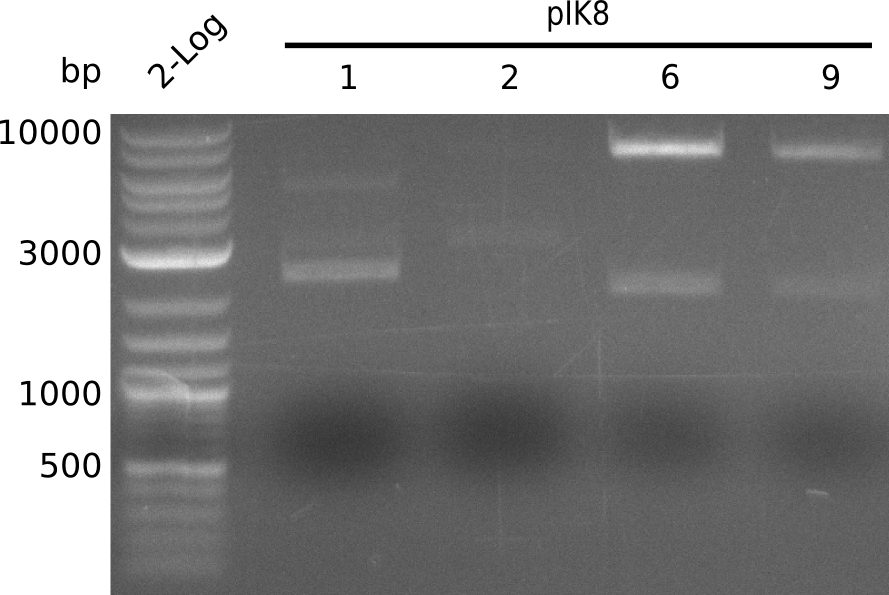
- very large and very small colonies present, pick starting with small colonies, run colony-PCR with primers IK26+VR (iTaq, 20 µl total volume) using 2 ng of BBa_I746200 in pSB1AK3 as positive control:
| Cycles | temperature [°C] | Time [s] |
|---|---|---|
| 1 | 95 | 300 |
| 35 | 95 | 30 |
| 54 | 30 | |
| 72 | 240 | |
| 1 | 72 | 600 |
| 1 | 10 | inf |
- clones grown in eppendorf tubes in 2xYT+Cm at 37°C
2013-09-14
- run gel
- colonies designated pIK8.1, pIK8.2, pIK8.6, pIK8.9 positive
- transfer to baci falcons, add 2xYT+Cm, grow at 37°C
2013-09-15
- make miniPreps: 73.5 ng/µl (pIK8.1), 12.9 ng/µl (pIK8.2), 289.0 ng/µl (pIK8.6), 169.0 ng/µl (pIK8.9) in 37.5 µl each
- digest with BamHI+HindIII using 2 µl (pIK8.1), 10 µl (pIK8.2), 1 µl (pIK8.6), 1 µl (pIK8.9) DNA (20 µl total volume)
- expected: 2.1 + 7.3 kb
 "
"