06/09/13
From 2013.igem.org
(Difference between revisions)
TanviSinha (Talk | contribs) |
TanviSinha (Talk | contribs) |
||
| Line 14: | Line 14: | ||
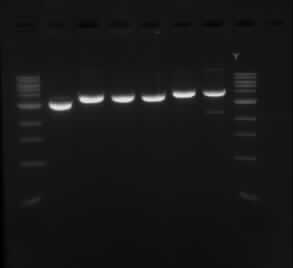
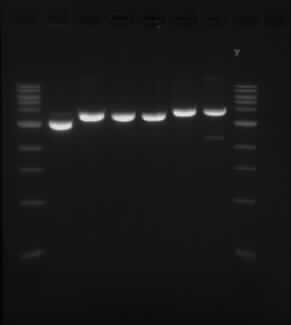
==Results from double digest of isolated plasmid with SpeI and XbaI== | ==Results from double digest of isolated plasmid with SpeI and XbaI== | ||
| - | [[05/09/13]] | + | Experiment conducted on the [[05/09/13]] |
| + | |||
[[File:tod_digestions_pgemvector_060913.jpg]] | [[File:tod_digestions_pgemvector_060913.jpg]] | ||
| Line 20: | Line 21: | ||
==Results from the transformation of pSB1C3/TOD genes== | ==Results from the transformation of pSB1C3/TOD genes== | ||
| - | [[05/09/13]] | + | Experiment conducted on the [[05/09/13]] |
| + | |||
| + | The positive RFP control showed colony growth on both the 20 ul and the 200 ul plates, but showed a mix of red and white colonies. | ||
| + | The background control also showed colony growth, which was not expected. Upon further investigation, it was discovered the XbaI and SpeI have complementary sticky ends which could result in re-circularisation of the vector backbone to itself. This explains the presence of white colonies on the RFP positive control and the growth on the background control. To identify if this has occurred, another double digest will be carried out. | ||
| + | |||
| + | The samples A (TodX), C (TodF) and E(ToBG) all showed colony growth for the 20 ul and the 200 ul plates. | ||
Revision as of 12:58, 6 September 2013
| Home | Team | Official Team Profile | Project | Parts Submitted to the Registry | Modeling | Notebook | Safety | Attributions |
|---|
Results from double digest of isolated plasmid with SpeI and XbaI
Experiment conducted on the 05/09/13
Results from the transformation of pSB1C3/TOD genes
Experiment conducted on the 05/09/13
The positive RFP control showed colony growth on both the 20 ul and the 200 ul plates, but showed a mix of red and white colonies. The background control also showed colony growth, which was not expected. Upon further investigation, it was discovered the XbaI and SpeI have complementary sticky ends which could result in re-circularisation of the vector backbone to itself. This explains the presence of white colonies on the RFP positive control and the growth on the background control. To identify if this has occurred, another double digest will be carried out.
The samples A (TodX), C (TodF) and E(ToBG) all showed colony growth for the 20 ul and the 200 ul plates.
 "
"