Team:BYU Provo/Notebook/SmallPhage/Winterexp/Period3/Exp/4.25 T7 phage viability test
From 2013.igem.org
(Difference between revisions)
| (6 intermediate revisions not shown) | |||
| Line 14: | Line 14: | ||
<font color="#333399" size="3" font face="Calibri"> | <font color="#333399" size="3" font face="Calibri"> | ||
| - | : | + | <font size = "4"> |
| + | |||
| + | : <u> '''Small Phage''' </u> </font> | ||
: [[Team:BYU Provo/Notebook/SmallPhage/Winterexp|March-April]] | : [[Team:BYU Provo/Notebook/SmallPhage/Winterexp|March-April]] | ||
| Line 38: | Line 40: | ||
: Perform T7+ titer to determine a workable concentration for selection test | : Perform T7+ titer to determine a workable concentration for selection test | ||
: Test phage viability in liquid culture | : Test phage viability in liquid culture | ||
| + | |||
'''II) Expected Outcome''' | '''II) Expected Outcome''' | ||
| Line 74: | Line 77: | ||
: iii) Incubate for almost two days (4.25 6:00pm – 4.27 2:00pm) | : iii) Incubate for almost two days (4.25 6:00pm – 4.27 2:00pm) | ||
: iv) The supernatant was collected, transferred to two 1.5mL centrifuge tubes, and stored in the fridge. | : iv) The supernatant was collected, transferred to two 1.5mL centrifuge tubes, and stored in the fridge. | ||
| + | |||
'''V) Results''' | '''V) Results''' | ||
| Line 112: | Line 116: | ||
2. Spot test results | 2. Spot test results | ||
| - | + | [[File:5.25SpotTest.JPG|400px|center]] | |
This is similar to what we saw before finals. This confirms that our phage dilution series have survived. Hurray! | This is similar to what we saw before finals. This confirms that our phage dilution series have survived. Hurray! | ||
| Line 118: | Line 122: | ||
3. Titer test result | 3. Titer test result | ||
| - | + | [[File:5.25T7Titer.JPG|400px|center]] | |
-5 and -6 will be a good place to start our selection test. We will also need to increase phage titer (phage concentration). | -5 and -6 will be a good place to start our selection test. We will also need to increase phage titer (phage concentration). | ||
| Line 124: | Line 128: | ||
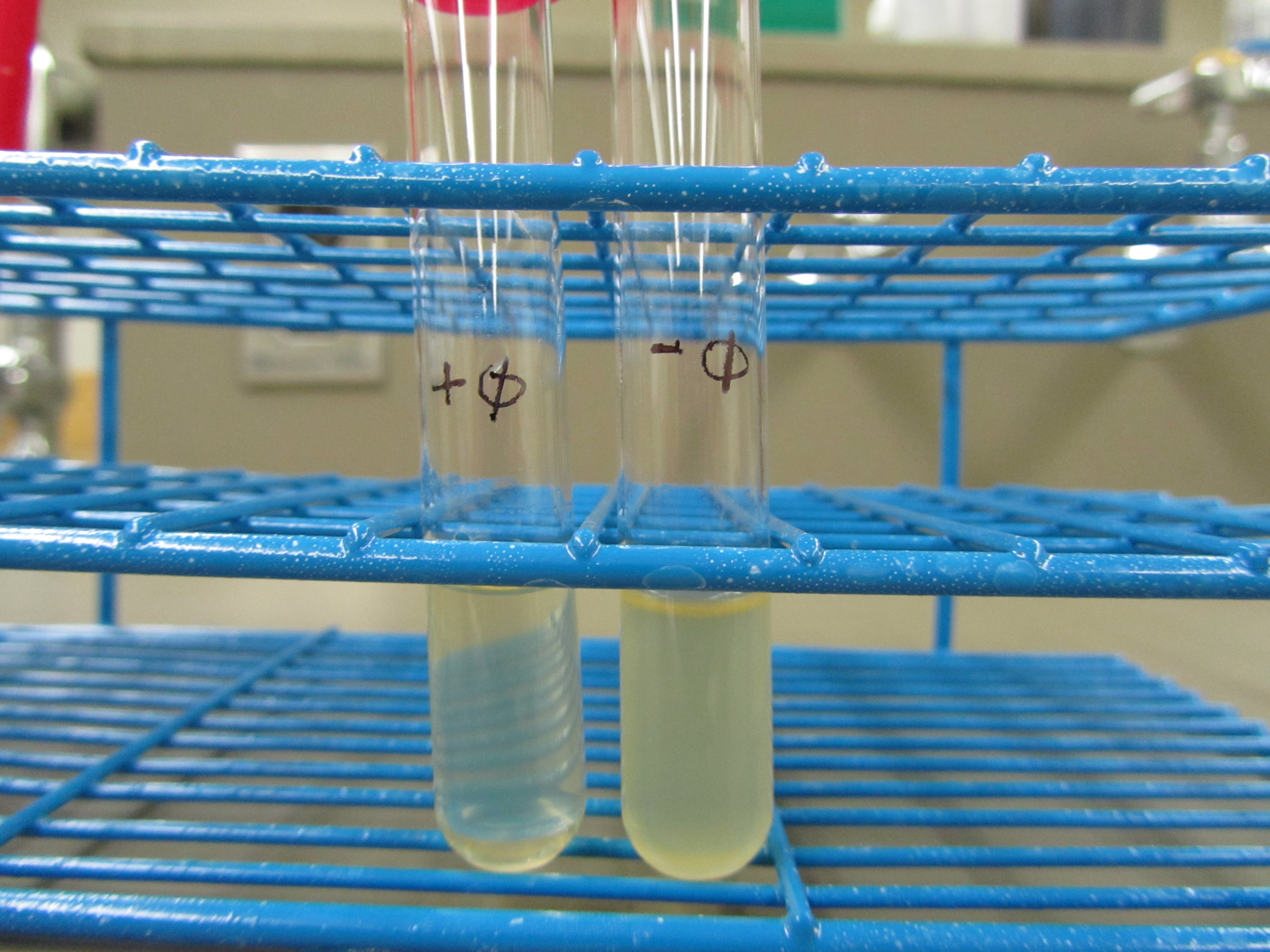
4. Liquid culture test (4.27 6:00pm) | 4. Liquid culture test (4.27 6:00pm) | ||
| - | + | [[File:SpringPR1P1.JPG|400px|center]] | |
Obvious clearage is seen in + phage test tube. E coli debris was also observed at the bottom of the + phage test tube. | Obvious clearage is seen in + phage test tube. E coli debris was also observed at the bottom of the + phage test tube. | ||
| + | |||
'''VI) Conclusion''' | '''VI) Conclusion''' | ||
| - | : T7+ have survived | + | : T7+ have survived for a week. We can continue to use the 4.8 dilution series for our experiments. This will save us some stock phage. |
: -5 is a good phage concentration to start our selection test. | : -5 is a good phage concentration to start our selection test. | ||
: The stock phage solution has a medium titer, we need to increase it to perform our mutation experiments. | : The stock phage solution has a medium titer, we need to increase it to perform our mutation experiments. | ||
| + | |||
'''VI) Proposed next step''' | '''VI) Proposed next step''' | ||
Latest revision as of 13:32, 9 September 2013
| Small Phage March - April Notebook: Experiments
| |||||||||||||||||||
|
|
4.25 T7 phage viability test
I) Purpose
1. Streak test for E coli BL21
2. Perform spot test
3. Perform titer test
4. Liquid culture test
1. Plates
2. Spot test results This is similar to what we saw before finals. This confirms that our phage dilution series have survived. Hurray! 3. Titer test result -5 and -6 will be a good place to start our selection test. We will also need to increase phage titer (phage concentration). 4. Liquid culture test (4.27 6:00pm) Obvious clearage is seen in + phage test tube. E coli debris was also observed at the bottom of the + phage test tube.
| ||||||||||||||||||
 "
"