Team:UNIK Copenhagen/Signe Notebook
From 2013.igem.org
Emilfischer (Talk | contribs) |
Emilfischer (Talk | contribs) |
||
| Line 374: | Line 374: | ||
Colonies 1, 5, 12 and 15 were inoculated to make liquid cultures, that were grown overnight. | Colonies 1, 5, 12 and 15 were inoculated to make liquid cultures, that were grown overnight. | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
</p> | </p> | ||
</div> | </div> | ||
| Line 386: | Line 381: | ||
<div class="left_page"> | <div class="left_page"> | ||
<h1>Week 9</h1> | <h1>Week 9</h1> | ||
| - | <p> | + | <p> |
| + | <u>Assembly of constructs</u> | ||
| + | <br> | ||
| + | Cultures of eFbFP-pJAM1786 was minipreped and samples were sent for sequencing. Microscopy revealed MamC-eGFP-pJAM1786 and eFbFP-pJAM1786 emitted fluorescence. To measure fluorescence quantitatively, we set up 8 liquid cultures of MamC-eGFP-pJAM1786 and 3 of MamC-pSB1C3 as a non-fluorescent control. Fluorescence was measured for dilutions 1X, 2X, 5X, 10X, 50X (Ex. 485 nm em. 535 nm.) using an ELISA reader (black plate) and gave rise characterization for BioBrick BBa_K1094401. | ||
| + | OD(600nm) was also measured to ensure approx. equal cell density. | ||
| + | <br><br> | ||
| + | |||
| + | <u>BioBrick submission</u> | ||
| + | <br> | ||
| + | Miniprep was performed and samples sent for sequencing. The sequencing results revealed that sequences of MamC-pSB1C3, eGFP(18)-pSB1C3 and eGFP(19)-pSB1C3 were correct while eFbFP-pSB1C3 held the wrong insert. We uncovered that this was due two "illegal" restriction sites in the FbFP sequence. We, therefore, ordered another synthetic gene from IDT with the restriction sites removed. | ||
| + | To submit MamC-eGFP as a BioBrick we PCR amplified (1:20 min elongation time) the whole construct (MamC F primer and eGFP R primer). To do this we used 3 different templates. | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | <br> | ||
| + | <a href="https://static.igem.org/mediawiki/2013/3/33/UNIK_Copenhagen_Notebook_ref_17.18.jpg" target="_blank"> | ||
| + | <img src="https://static.igem.org/mediawiki/2013/3/33/UNIK_Copenhagen_Notebook_ref_17.18.jpg" height="120"> | ||
| + | </a> | ||
| + | <br> | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | </p> | ||
</div> | </div> | ||
<div class="right_page"> | <div class="right_page"> | ||
| Line 396: | Line 418: | ||
<div class="left_page"> | <div class="left_page"> | ||
<h1>Week 10</h1> | <h1>Week 10</h1> | ||
| - | <p> | + | <p> |
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | </p> | ||
</div> | </div> | ||
<div class="right_page"> | <div class="right_page"> | ||
Revision as of 06:51, 22 September 2013
Notebook
Get our Nootebook in a printable version here .
Week 1 - July 8th-14th
Culturing of magnetotactic bacteria (MTBs)
Making base medium for growing magnetotactic bacteria. This medium was also used to make agar plates.
MS-1 inoculated in liquid base medium. Falcon tubes were flushed with the nitrogen before use and incubated at 28 degrees.
Plates were kept in a closed container with an Oxoid AnaeroGen pad.
Assembly of constructs
Glycerol stock of E. coli with pBBR1MCS-2 was thawed to grow on these plates.
Primers for MamC (MS-1 strain) was ordered.
Week 3
Culturing of MTBs
making charcoal media and plates. MS-1 and MSR-1 was inoculated. Liquid cultures were setup in Hungate tubes 200ul, 400ul and 600ul of each strain (MS-1 and MSR-1). Charcoal plates were inoculated as well with both strains.
Assembly of constructs
Colony PCRs of eFbFP-pEX-A and eGFP-pDRIVE. Only eFbFP was successful.

Overnight cultures were made of colonies 1-4 (eFbFP-pEX-A). Miniprep of eFbFP-pEX-A
cultures. Samples sent for sequencing.
Overnight cultures were made of colonies 1-4 (eFbFP-pEX-A).
Miniprep of eFbFP-pEX-A cultures. Samples sent for sequencing.
Colony PCR of eGFP-pDRIVE was repeated. Still negative.
Cloning and transformation was also repeated. CloneJET PCR cloning kit was used to create eGFP-pJET (pJET was chosen instead of pDRIVE). Not successful.
Gradient PCR of eGFP premade vector (56-65 degrees). Gel electrophoresis was carried out and the bands were purified from the gel.
An attempt to clone eGFP into pDRIVE was not successful.
The cloning of eFbFP into expression vector pBBR1MCS-2 was successful instead.
Colony PCR of eGFP-pDRIVE and eFbFP-pBBR1MCS-2. Gel electrophoresis revealed only one colony to be successful (eFbFP-pBBR1MCS-2 no. 17).
Week 4
Assembly of constructs
Colony PCR of eGFP-pDRIVE was repeated with increased elongation time (1 min.)
 Cloning looks successful and colonies 1-4 were set up as liquid cultures overnight.
Cloning looks successful and colonies 1-4 were set up as liquid cultures overnight.
Colony PCR of eFbFP-pBBR1MCS-2 because only one colony was positive in week 3. Increased elongation time.
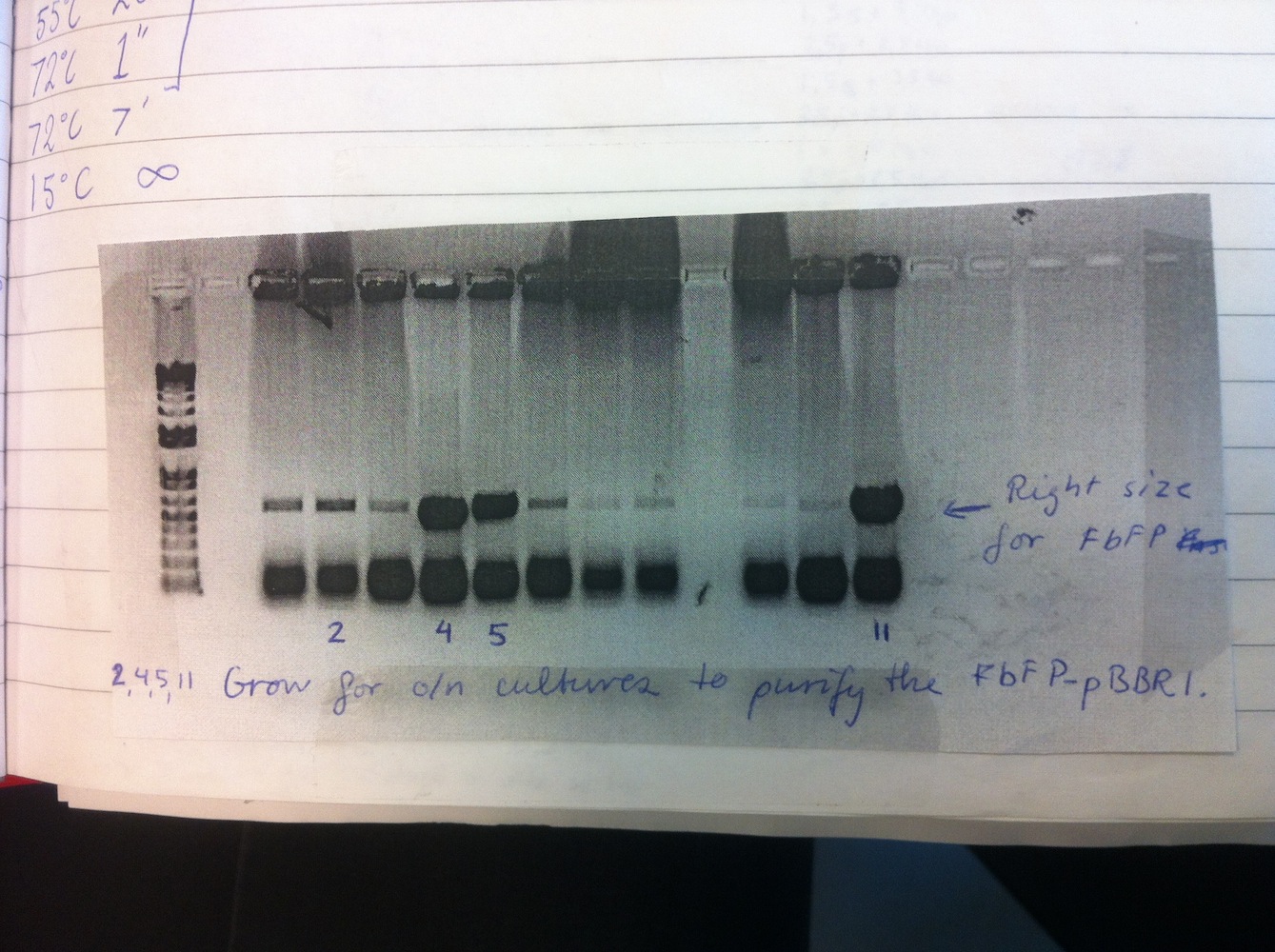
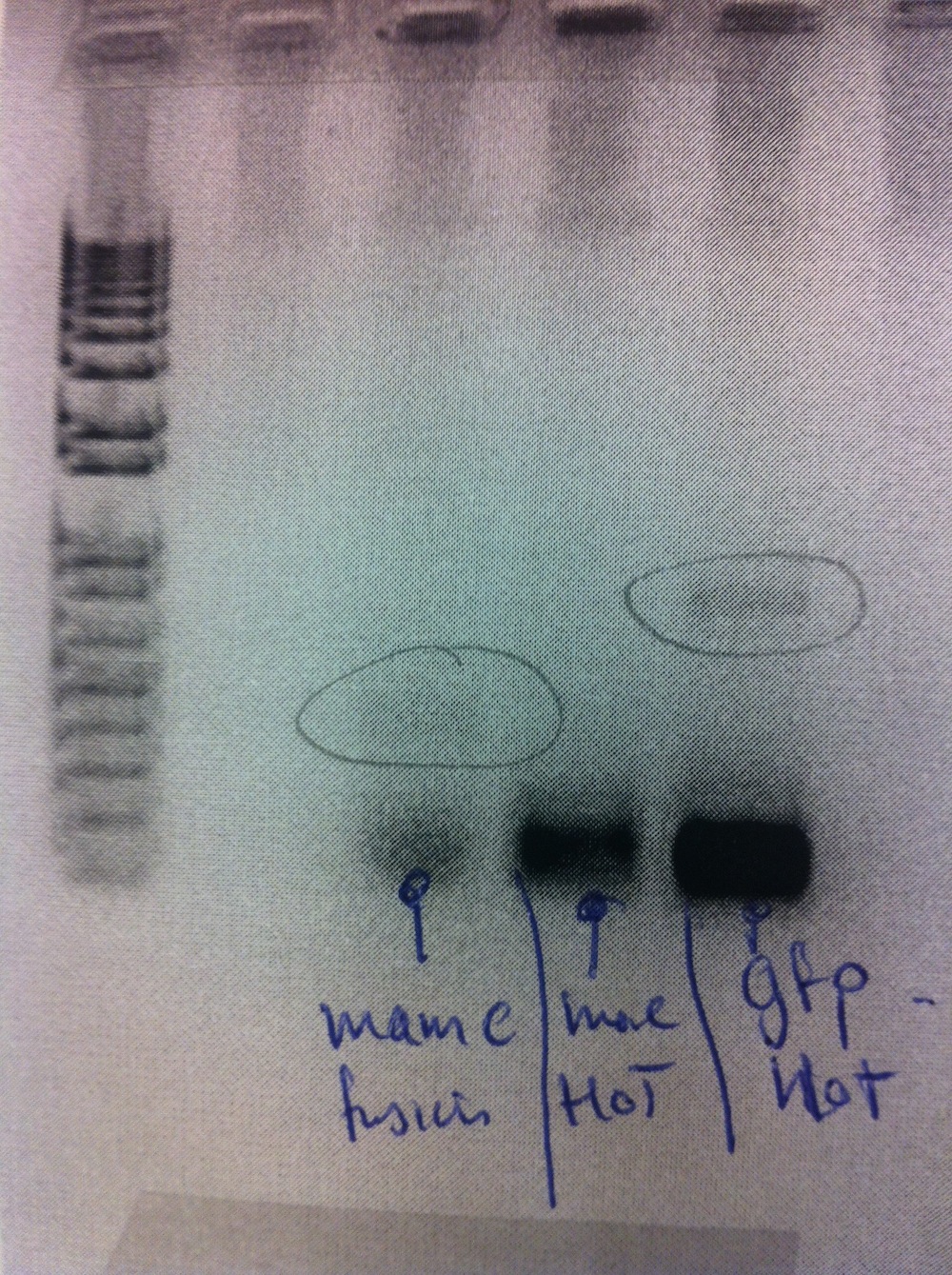
Overnight liquid cultures were setup for colonies 2, 4, 5 and 11.
Miniprep was performed on these cultures alongside eGFP-pDRIVE colonies 1-4. This gave good yields and the plasmids were sent for sequencing. This showed eGFP-pDRIVE no. 4 and eFbFP-pBBR1MCS-2 no. 17 was correct!
Cloning and transformation of eGFP-pBBR1MCS-2 using plasmid no. 4 (mentioned just above). The transformation only gave rise to 2 colonies, thus suggesting the cloning to be unsuccessful. The cloning was repeated and transformation was carried out again. Colony PCR was performed alongside MamC-pJET (see gel below).
Liquid cultures were setup from eFbFP-pBBR1MCS-2 no. 2, 4, 5, 11, 17 for 2 hours in order to measure fluorescence. First, OD(600nm) was measured to ensure equal density of cells. The measurements did not show fluorescence (ex. 450 nm, em. 495 nm) from the cultures.
Cloning and transformation of MamC-pDRIVE. Colony PCR showed the cloning to be of cryptic success. Colony PCR was redone without success.
Cloning was repeated using the CloneJET PCR cloning kit. MamC-pJET was transformed and plated. Colony PCR was carried out alongside eGFP-pBBR1MCS-2.
 The gel electrophoresis indicated MamC-pJET to be successfully cloned. However, only a few of the eGFP-pBBR1MCS-2 colonies appeared to have contain the insert.
The gel electrophoresis indicated MamC-pJET to be successfully cloned. However, only a few of the eGFP-pBBR1MCS-2 colonies appeared to have contain the insert.
Week 5
Culturing of MTBs
Colony PCR using MamC primer was done with MS-1 and MSR-1 colonies. MamC was amplified from the genomic DNA. This did not give rise to any bands via gel electrophoresis.
MamC was, however, successfully amplified from MSR-1 later in the week (see gel below).

Assembly of constructs
Colonies 1, 2, 3 (MamC-pJET) and II, IV, VIII (eGFP-pBBR1MCS-2) were setup in liquid cultures (see colony PCR from previous week). Miniprep was carried out and plasmids were sent for sequencing. All MamC sequences were perfect while all eGFP sequences were wrong. The cloning of eGFP into pDRIVE was successfully redone (documentation lost). Fluorescence was also measured of eFbFP-pBBR1MCS-2 strains. No fluorescence detected as the sequencing explains. Inducing with IPTG (final conc. 1 mM) had no effect.
The CPH strain
Enrichment of magnetotactic bacteria was done according to the protocol. It was unclear whether the bacteria observed in the microscope were actually magnetotactic. They were compared to MS-1 and MSR-1 samples.
Week 6
Assembly of constructs
Since we couldn't detect any fluorescence from eFbFP-pBBR1MCS-2 we needed troubleshoot this. We found a frameshift in the vector and thus we repeated the cloning of eFbFP into pBBR1MCS-2. This was done alongside cloning eGFP into pBBR1MCS-2. Transformation and colony PCR were carried out. All colonies appeared to be positive on the gel electrophoresis (see picture below).

MamC-pJET did not contain the restriction sites we needed. Therefore, the whole procedure for obtaining MamC-pJET was repeated using other primers (with different overhangs).
Six of each genotype were set up for liquid cultures overnight (colonies 1, 2, 6, 9, 13, 16 for eFbFP and 18, 19, 23, 25, 27, 31 for eGFP). Miniprep was done and samples were sent for sequencing. None of the eFbFP gave rise to good quality sequencing. Colonies 18, 19, 23 and 27 of eGFP contained the correct sequence.
Empty pBBR1MCS-2 vector was transformed into E. cloni in order to obtain more stock vector.
Culturing of MTBs
MSR-1 base medium culture was inoculated in new medium and put in 28 degrees shaker over the weekend. This was done to check whether the strain would grow aerobically as well.
Week 7
more text on this page!
Week 8
Assembly of constructs
Sequencing of eFbFP-pDONR207 and MamC-eGFP-pDONR207. Gateway LR reaction for cloning MamC-eGFP (no 11 and no 13) into pJAM1786 and transformation into E. coli.
Colony PCR was performed on MamC-eGFP-pJAM1786 and eFbFP-pDONR207 (see gel below)

Colonies 1, 2, 6, 10, 11, 13, 14, 16 were inoculated in liquid culture and grown overnight. Miniprep was performed and LR reaction was done using eFbFP-pDONR207 no 11. Enhanced FbFP-pJAM1786 was transformed and grown overnight.
Expressing eFbFP in the gateway system was repeated. PCR amplification using Gateway primers
on eFbFP-pEX-A was carried out. Gateway BP reaction was done to move the fragment into pDONR207. Afterwards the plasmid was transformed into E. coli.
The CPH strain
Cloning of 16S PCR products into pJET and subsequent transformation into E. coli. Liquid cultures were grown inoculating cells from 24 different colonies (12 from H.C. Ørsted Parken sample and 12 from Christiania sample). Miniprep was carried out and samples were sent for sequencing.
BioBrick submission
Colony PCR was performed.
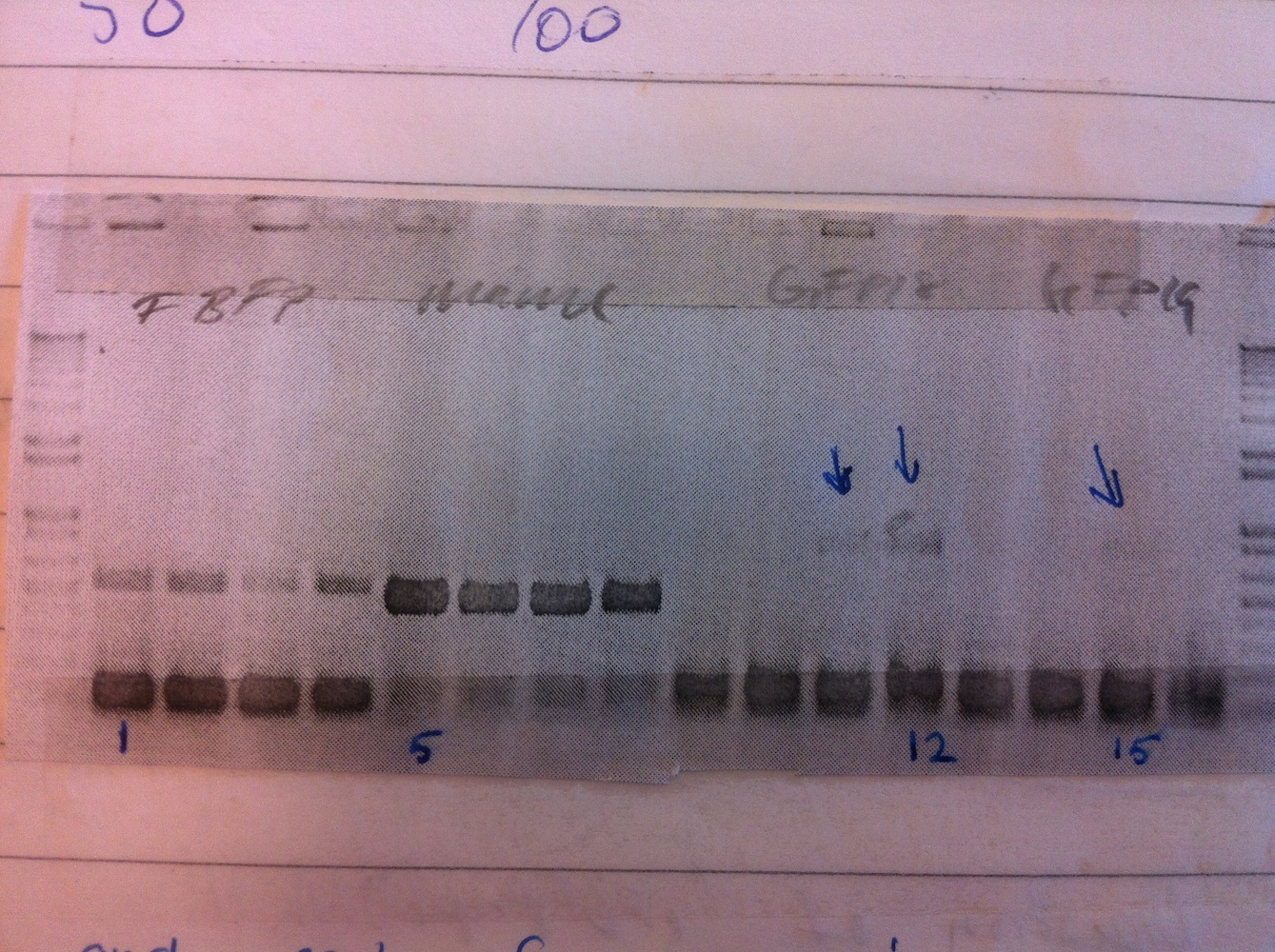 Colonies 1, 5, 12 and 15 were inoculated to make liquid cultures, that were grown overnight.
Colonies 1, 5, 12 and 15 were inoculated to make liquid cultures, that were grown overnight.
Week 9
Assembly of constructs
Cultures of eFbFP-pJAM1786 was minipreped and samples were sent for sequencing. Microscopy revealed MamC-eGFP-pJAM1786 and eFbFP-pJAM1786 emitted fluorescence. To measure fluorescence quantitatively, we set up 8 liquid cultures of MamC-eGFP-pJAM1786 and 3 of MamC-pSB1C3 as a non-fluorescent control. Fluorescence was measured for dilutions 1X, 2X, 5X, 10X, 50X (Ex. 485 nm em. 535 nm.) using an ELISA reader (black plate) and gave rise characterization for BioBrick BBa_K1094401.
OD(600nm) was also measured to ensure approx. equal cell density.
BioBrick submission
Miniprep was performed and samples sent for sequencing. The sequencing results revealed that sequences of MamC-pSB1C3, eGFP(18)-pSB1C3 and eGFP(19)-pSB1C3 were correct while eFbFP-pSB1C3 held the wrong insert. We uncovered that this was due two "illegal" restriction sites in the FbFP sequence. We, therefore, ordered another synthetic gene from IDT with the restriction sites removed.
To submit MamC-eGFP as a BioBrick we PCR amplified (1:20 min elongation time) the whole construct (MamC F primer and eGFP R primer). To do this we used 3 different templates.

more text on this page!
Week 10
more text on this page!
Week 11
This week the team...
more text on this page!
Week 12
This week the team...
more text on this page!
 "
"