Team:BYU Provo/Notebook/LargePhage/Winterexp/Period3/Dailylog
From 2013.igem.org
(Created page with "{{TeamBYUProvo}} <br> {| width="100%" | colspan="3" | <font color="#333399" size="5" font face="Calibri"> '''Large Phage March - April Notebook: March 15 - March 31 Daily Log''...") |
|||
| (3 intermediate revisions not shown) | |||
| Line 4: | Line 4: | ||
{| width="100%" | {| width="100%" | ||
| - | | colspan="3" | <font color="#333399" size="5" font face="Calibri"> '''Large Phage March - April Notebook: | + | | colspan="3" | <font color="#333399" size="5" font face="Calibri"> '''Large Phage March - April Notebook: April 1- April 14 Daily Log'''</font> |
<br> | <br> | ||
| Line 14: | Line 14: | ||
<font color="#333399" size="3" font face="Calibri"> | <font color="#333399" size="3" font face="Calibri"> | ||
| - | : | + | <font size="4"> |
| + | |||
| + | : <u> '''Large Phage''' </u> </font> | ||
: [[Team:BYU Provo/Notebook/LargePhage/Winterexp|March-April]] | : [[Team:BYU Provo/Notebook/LargePhage/Winterexp|March-April]] | ||
| Line 30: | Line 32: | ||
<font face="Calibri" size="3"> | <font face="Calibri" size="3"> | ||
| - | <font size="4"> ''' | + | <font size="4"> '''4/1/13''' </font> |
| - | + | 4/1/13 KS &BDM | |
| - | + | Today we did presentations. I talked about DNA packaging in phage. Right now we’re waiting for our phage to come in so we can do some mutagenesis! | |
| - | for | + | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | <font size="4"> '''4/3/13''' </font> | |
| - | + | 4/3/13 KS & BDM | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | 3 | + | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| + | Final Project notes: | ||
| + | 1. Watch a science talk and write a paragraph about the presentation. Write a one paragraph critique. | ||
| + | T/TH 11:00 and 4:00 | ||
| + | 2. Give a presentation on our project so far (15 minutes) | ||
| + | 5-10 minutes on the Background | ||
| + | -Hypothesis, what is our question, why are we researching this | ||
| + | 5-10 minutes methods and results | ||
| + | Conclusion- Short, clear bulleted | ||
| - | + | Things we need: | |
| - | + | H-Broth | |
| - | + | adenine (20ug/ml) | |
| - | + | e.coli B bacteria | |
| + | m9+ medium | ||
| + | uricil | ||
| + | 200ug 5-bromodeoxyuridine, 20ug uridine, 20ug adenine per ml | ||
| + | *all work was done in yellow light? | ||
| + | chloroform | ||
| + | calcium chloride | ||
| + | magnesium chloride | ||
| + | To do: grow up T1 and T4 | ||
| - | + | <font size="4"> '''4/5/13''' </font> | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | 4/5/13-KS and BDM | |
| - | + | Today we did a spot test with 11T4, T4Do(s), 12T4r, 40T4r, 40T4 with e.coli BL21. | |
| - | + | Procedure: | |
| - | + | We plated .5 mL of an overnight culture of E. coli BL21 in 4.5 mL of LB 1x top agar. When it had hardened, we spot tested 5 uL of the following samples on the plates: 11T4, T4Do(s), 12T4r, 40T4r, 40T4 into 6 sectors of a plate. T4Do came from Dr. Casjens at the University of Utah. We incubated these plates at 37 degrees Celsius. | |
| - | + | We plated a control of just bacteria. | |
| - | + | Also, we infected .5 mL of BL21 with 1 uL of the T4Do sample for 20 mins, and then plated it. | |
| + | We started two liquid cultures, each containing 1 mL of BL21. One received 1 uL of T4Do, the other received 10 uL. These were incubated on a shaker at 37C. | ||
| - | + | <font size="4"> '''4/8/13''' </font> | |
| - | + | ||
| + | 4/8/1/3-BDM and KS | ||
| + | [[File:April8pic1.jpg|400px]] | ||
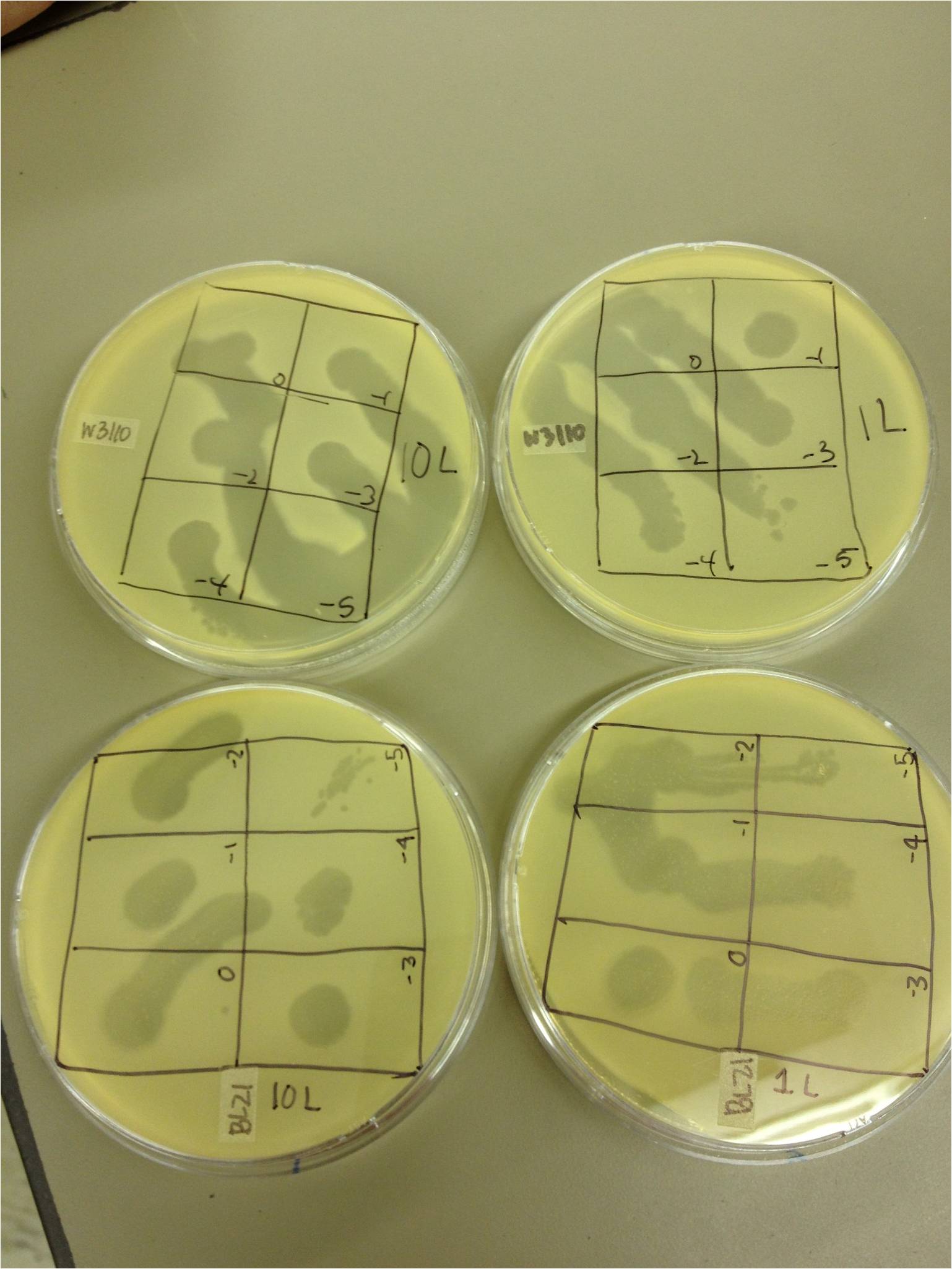
| - | + | We performed a phage titer spot test on two bacteria. We used the BL21 and W3110 strains of E. coli. We first put 100 microL of broth into epindorf tubes. We then took 10 microL of 1L, 10L, 40T4, T4DOS, and T4 infected phage and placed it in separate tubes labeled -1. We performed a dilution series taking out 10 microL each time. The last tube we did not remove 10 microL so the total volume was 110 microL. Next we added 4.5 mL of 1x top agar to .5mL of bacteria for both strains and plated it on LB plates. We had previously divided the plates into six sections with the labels 0, -1, -2, -3, -4, and -5. We took the phage from each concentration and spotted it on the plates. We allowed the plates to sit and let the phage soak in. We then placed the plates in the 37 C overnight. | |
| + | <font size="4"> '''4/10/13''' </font> | ||
| + | Results (Wed. 4/10/13) | ||
| + | We saw phage plaques in all spots (10^0 through 10^-5) on all bacteria for 10L (10uL of T4Do stock in 1 mL of bacteria grown over the weekend), 1L (1uL same as above), T4Do (stock), and T4 infected plate flood. 40T4 from the fridge grew on BL21 but not W3110. | ||
| + | I had started two liquid cultures of 100uL of liquid from the plate flood in 1mL of W3110 and BL21. The W3110 bacteria was visibly lysed (clear); the BL21 bacteria had some snot but was not lysed. | ||
| - | + | Procedure | |
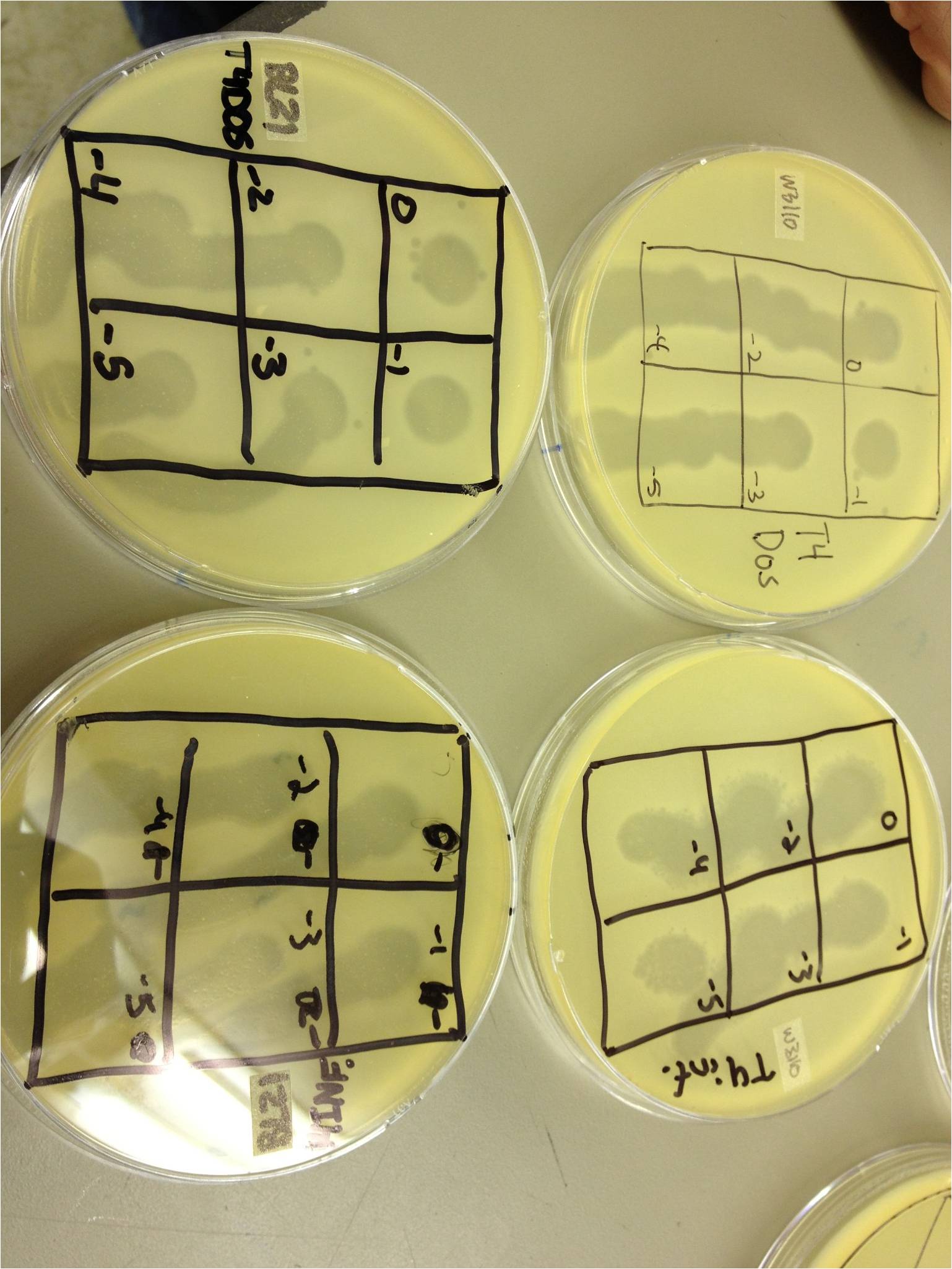
| + | We pelleted the liquid from the two liquid cultures and did a 10^0 through 10^-5 dilution on each, along with a bacterial lawn control. | ||
| + | We also started two more liquid cultures of 1 mL from the plate flood in 10 mL of W3110. We will shake these in a 100 mL flask at 37C. | ||
| + | [[File:Example2.jpg|400px]] | ||
| + | [[File:Example3.jpg|400px]] | ||
| + | [[File:Example4.jpg|400px]] | ||
| + | <font size="4"> '''4/12/13''' </font> | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
� | � | ||
| - | + | 12 April 2013 - Friday - BDM and KS | |
| - | + | The liquid culture titers from last time worked well--there were spots down to 10^-5. | |
| - | + | The liquid culture flasks just looked like normal bacteria--there was no visible lysis. | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
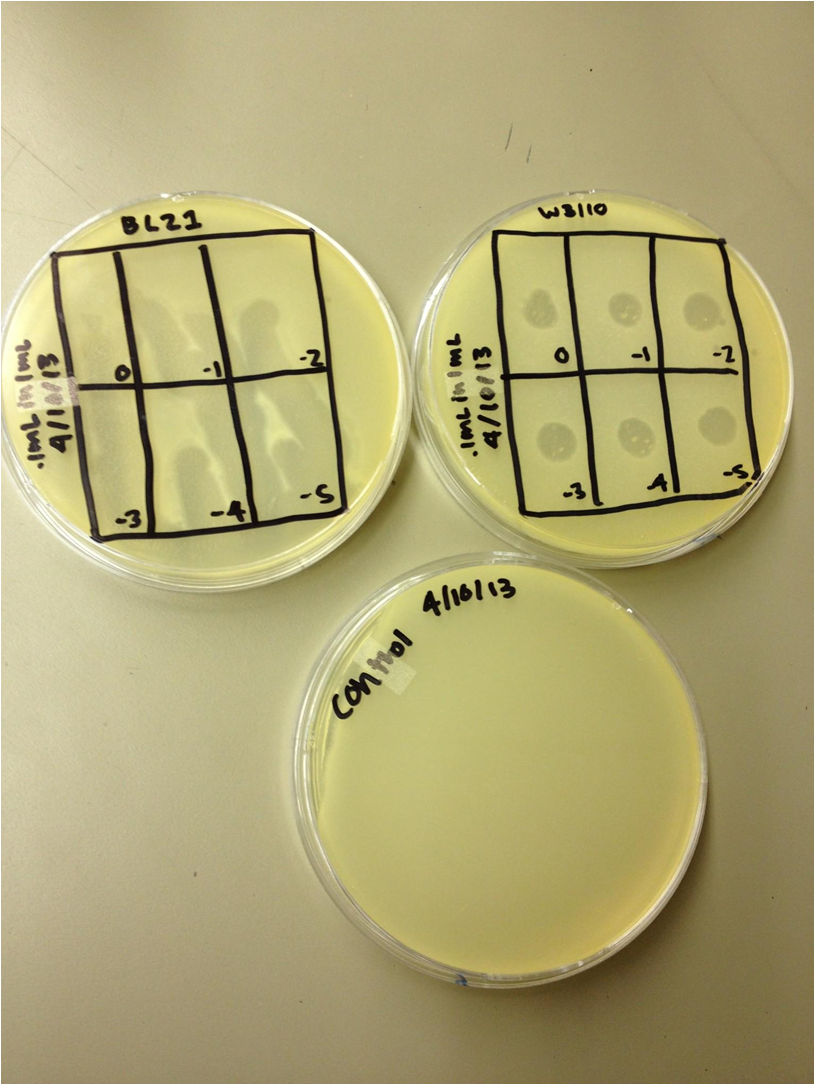
| - | + | Today we pelleted and filtered the liquid cultures and worked on our presentations for Monday. | |
| - | + | [[File:Example5.jpg|400px]] | |
<br> | <br> | ||
Latest revision as of 17:49, 26 September 2013
| Large Phage March - April Notebook: April 1- April 14 Daily Log
| ||
|
|
4/1/13 4/1/13 KS &BDM Today we did presentations. I talked about DNA packaging in phage. Right now we’re waiting for our phage to come in so we can do some mutagenesis! 4/3/13 4/3/13 KS & BDM Final Project notes: 1. Watch a science talk and write a paragraph about the presentation. Write a one paragraph critique. T/TH 11:00 and 4:00 2. Give a presentation on our project so far (15 minutes) 5-10 minutes on the Background -Hypothesis, what is our question, why are we researching this 5-10 minutes methods and results Conclusion- Short, clear bulleted Things we need: H-Broth adenine (20ug/ml) e.coli B bacteria m9+ medium uricil 200ug 5-bromodeoxyuridine, 20ug uridine, 20ug adenine per ml
chloroform calcium chloride magnesium chloride To do: grow up T1 and T4 4/5/13 4/5/13-KS and BDM Today we did a spot test with 11T4, T4Do(s), 12T4r, 40T4r, 40T4 with e.coli BL21. Procedure: We plated .5 mL of an overnight culture of E. coli BL21 in 4.5 mL of LB 1x top agar. When it had hardened, we spot tested 5 uL of the following samples on the plates: 11T4, T4Do(s), 12T4r, 40T4r, 40T4 into 6 sectors of a plate. T4Do came from Dr. Casjens at the University of Utah. We incubated these plates at 37 degrees Celsius. We plated a control of just bacteria. Also, we infected .5 mL of BL21 with 1 uL of the T4Do sample for 20 mins, and then plated it. We started two liquid cultures, each containing 1 mL of BL21. One received 1 uL of T4Do, the other received 10 uL. These were incubated on a shaker at 37C. 4/8/13 4/8/1/3-BDM and KS 400px We performed a phage titer spot test on two bacteria. We used the BL21 and W3110 strains of E. coli. We first put 100 microL of broth into epindorf tubes. We then took 10 microL of 1L, 10L, 40T4, T4DOS, and T4 infected phage and placed it in separate tubes labeled -1. We performed a dilution series taking out 10 microL each time. The last tube we did not remove 10 microL so the total volume was 110 microL. Next we added 4.5 mL of 1x top agar to .5mL of bacteria for both strains and plated it on LB plates. We had previously divided the plates into six sections with the labels 0, -1, -2, -3, -4, and -5. We took the phage from each concentration and spotted it on the plates. We allowed the plates to sit and let the phage soak in. We then placed the plates in the 37 C overnight. 4/10/13 Results (Wed. 4/10/13) We saw phage plaques in all spots (10^0 through 10^-5) on all bacteria for 10L (10uL of T4Do stock in 1 mL of bacteria grown over the weekend), 1L (1uL same as above), T4Do (stock), and T4 infected plate flood. 40T4 from the fridge grew on BL21 but not W3110. I had started two liquid cultures of 100uL of liquid from the plate flood in 1mL of W3110 and BL21. The W3110 bacteria was visibly lysed (clear); the BL21 bacteria had some snot but was not lysed. Procedure We pelleted the liquid from the two liquid cultures and did a 10^0 through 10^-5 dilution on each, along with a bacterial lawn control. We also started two more liquid cultures of 1 mL from the plate flood in 10 mL of W3110. We will shake these in a 100 mL flask at 37C. 4/12/13 � 12 April 2013 - Friday - BDM and KS The liquid culture titers from last time worked well--there were spots down to 10^-5. The liquid culture flasks just looked like normal bacteria--there was no visible lysis. Today we pelleted and filtered the liquid cultures and worked on our presentations for Monday.
| |
 "
"