Team:Tsinghua-E/Part1
From 2013.igem.org
(Created page with "{{Team:Tsinghua-E/TemplateTeam}} <html xmlns="http://www.w3.org/1999/xhtml"> <style type="text/css"> #content{height:2760px;} p{font-size:120%} .memberx {position:absolute;top:60...") |
|||
| (29 intermediate revisions not shown) | |||
| Line 2: | Line 2: | ||
<html xmlns="http://www.w3.org/1999/xhtml"> | <html xmlns="http://www.w3.org/1999/xhtml"> | ||
<style type="text/css"> | <style type="text/css"> | ||
| - | #content{height: | + | #content{height:1800px;} |
| - | p{font-size: | + | p{font-size:130%} |
.memberx {position:absolute;top:60px;left:5px;height:215px;width;300px;} | .memberx {position:absolute;top:60px;left:5px;height:215px;width;300px;} | ||
.memberx span{z-index:50;position:relative;width:453px;display:block;top:-215px;left:305px;color:#000000} | .memberx span{z-index:50;position:relative;width:453px;display:block;top:-215px;left:305px;color:#000000} | ||
| Line 10: | Line 10: | ||
<div style="position:absolute;top:176px;left:56px;width:105px;height:186px;z-index:99;"> | <div style="position:absolute;top:176px;left:56px;width:105px;height:186px;z-index:99;"> | ||
<ul class="leftdh"> | <ul class="leftdh"> | ||
| - | <li><a href=" | + | <li><a href="https://2013.igem.org/Team:Tsinghua-E/Part1" id="partslink">Part1</a></li> |
| - | <li><a href=" | + | <li><a href="https://2013.igem.org/Team:Tsinghua-E/Part2" id="partslink">Part2</a></li> |
| - | <li><a href=" | + | <li><a href="https://2013.igem.org/Team:Tsinghua-E/Part3" id="partslink">Part3</a></li> |
| - | <li><a href=" | + | <li><a href="https://2013.igem.org/Team:Tsinghua-E/Part4" id="partslink">Part4</a></li> |
</ul> | </ul> | ||
</div> | </div> | ||
| - | <div class="tmc" style="height: | + | <div class="tmc" style="height:1685px;"> |
</div> | </div> | ||
| - | <div class="neirong" style="height: | + | <div class="neirong" style="height:1665px;"> |
| + | </html> | ||
| - | < | + | <h2>[[File:4_1s.png|68px|left]] '''Part 1: THU-E Mutation Part''' </h2> |
| - | + | [[File:Plasmid1.png|446px|right]] | |
| - | + | ||
| - | + | A plasmid used for the construction of high-diversity library in vivo ingenome level. In this vector, highly error-prone <em>dnaQ</em> mutant, <em>mutD</em><html><a href="#_ENREF_1" title="Lou, 2012 #499">[1]</a></html>was cloned downstream of <em>araBAD</em> promoter to control the mutation rate of the target genome by the concentration of <em>araBAD</em> promoter’s inducer, L-arabinose, in a strict manner.<em>E. Coli</em> JM109 carrying different vectors of pBAD_B0030-<em>mutD</em>-<em>sfGFP</em>, pBAD_B0032-<em>mutD</em>-<em>sfGFP</em> and pBAD_SDA_RBS-<em>mutD-sfGFP</em>(this RBS sequence was derived from the RBS sequence upstream of sfGFP in original AraC_pBAD_CI_OR222-sfGFP vector<html><a href="#_ENREF_2" title="Lou, 2012 #499">[2]</a></html>)were constructed. By detecting the induced fluorescence intensity, we found that pBAD_B0030-<em>mutD</em>-<em>sfGFP</em>, andpBAD_SDA_RBS-<em>mutD- sfGFP</em>have relatively higher <em>mutD</em> expression. The increaseof mutation rate induced by our mutation part was measured by quantifying the reversion of rifampinresistance caused by mutation in genome.pBAD_SDA_RBS-<em>mutD- sfGFP</em>could increase the genome mutation rate up to 10 times compared with negative control with 1g/L induction concentration of L-arabinose. <br /> | |
| + | |||
| + | <html> | ||
<p align="center"><br /> | <p align="center"><br /> | ||
| - | <img border="0" width="446 | + | <img border="0" width="446" src="/wiki/images/a/a3/Part1II.png" /> <br /> |
| - | Figure.1 | + | Figure.1 Conception illustration of the working mechanism of <em>mutD</em> |
| - | <img border="0" width=" | + | <img border="0" width="446" src="/wiki/images/7/70/Part1I.png" /> <br /> |
Figure.2 rifampicin reversion mutants caused by <em>mutD</em> expression and the counts by agar plate<br /> | Figure.2 rifampicin reversion mutants caused by <em>mutD</em> expression and the counts by agar plate<br /> | ||
| - | + | </p> | |
| - | + | <br/> | |
| + | <br/> | ||
| + | <p><font style="font-size: 12px">[1] Schaaper, R. M. Mechanisms of mutagenesis in the Escherichia-Coli mutator mutd5 - role of DNA mismatch repair. <em>Proc. Natl. Acad. Sci. U. S. A.</em> 85, 8126-8130,doi:10.1073/pnas.85.21.8126 (1988).</font></p> | ||
| + | <p><font style="font-size: 12px">[2] Lou, C. B., Stanton, B., Chen, Y. J., Munsky, B. & Voigt, C. A. Ribozyme-based insulator parts buffer synthetic circuits from genetic context. <em>Nature Biotechnology</em> 30, 1137-+, doi:10.1038/nbt.2401 (2012).</font></p> | ||
| + | </div> | ||
</html> | </html> | ||
Latest revision as of 03:07, 28 September 2013



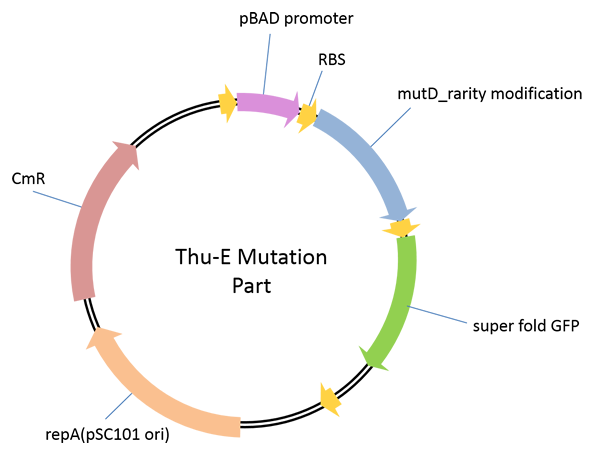
Part 1: THU-E Mutation Part
A plasmid used for the construction of high-diversity library in vivo ingenome level. In this vector, highly error-prone dnaQ mutant, mutD[1]was cloned downstream of araBAD promoter to control the mutation rate of the target genome by the concentration of araBAD promoter’s inducer, L-arabinose, in a strict manner.E. Coli JM109 carrying different vectors of pBAD_B0030-mutD-sfGFP, pBAD_B0032-mutD-sfGFP and pBAD_SDA_RBS-mutD-sfGFP(this RBS sequence was derived from the RBS sequence upstream of sfGFP in original AraC_pBAD_CI_OR222-sfGFP vector[2])were constructed. By detecting the induced fluorescence intensity, we found that pBAD_B0030-mutD-sfGFP, andpBAD_SDA_RBS-mutD- sfGFPhave relatively higher mutD expression. The increaseof mutation rate induced by our mutation part was measured by quantifying the reversion of rifampinresistance caused by mutation in genome.pBAD_SDA_RBS-mutD- sfGFPcould increase the genome mutation rate up to 10 times compared with negative control with 1g/L induction concentration of L-arabinose.

Figure.1 Conception illustration of the working mechanism of mutD
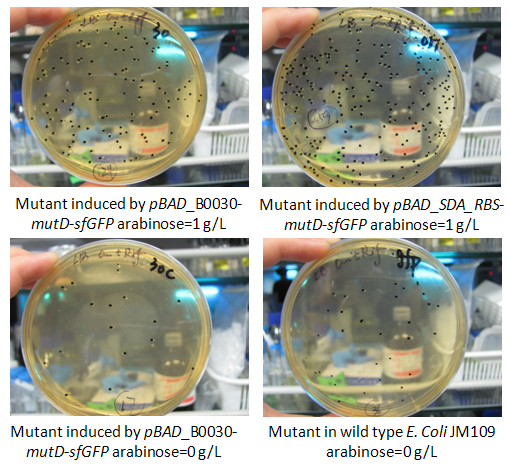
Figure.2 rifampicin reversion mutants caused by mutD expression and the counts by agar plate
[1] Schaaper, R. M. Mechanisms of mutagenesis in the Escherichia-Coli mutator mutd5 - role of DNA mismatch repair. Proc. Natl. Acad. Sci. U. S. A. 85, 8126-8130,doi:10.1073/pnas.85.21.8126 (1988).
[2] Lou, C. B., Stanton, B., Chen, Y. J., Munsky, B. & Voigt, C. A. Ribozyme-based insulator parts buffer synthetic circuits from genetic context. Nature Biotechnology 30, 1137-+, doi:10.1038/nbt.2401 (2012).
 "
"